Three pulse recoupling and phase jump matching
- PMID: 26777742
- PMCID: PMC4959450
- DOI: 10.1016/j.jmr.2015.11.012
Three pulse recoupling and phase jump matching
Abstract
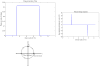
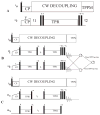
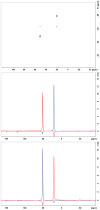
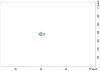
The paper describes a family of novel recoupling pulse sequences, called three pulse recoupling. These pulse sequences can be employed for both homonuclear and heteronuclear recoupling experiments and are robust to dispersion in chemical shifts and rf-inhomogeneity. These recoupling pulse sequences can be used in design of two-dimensional solid state NMR experiments that use powdered dephased antiphase coherence (γ preparation) to encode chemical shifts in the indirect dimension. Both components of this chemical shift encoded gamma-prepared states can be refocused into inphase coherence by a recoupling element. This helps to achieve sensitivity enhancement in 2D NMR experiments by quadrature detection.
Keywords: Gamma preparation; Hartmann–Hahn matching; MAS; Quadrature detection; Recoupling.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Recoupling in solid state NMR using γ prepared states and phase matching.J Magn Reson. 2011 Oct;212(2):402-11. doi: 10.1016/j.jmr.2011.07.024. Epub 2011 Aug 11. J Magn Reson. 2011. PMID: 21889380 Free PMC article.
-
Two pulse recoupling.J Magn Reson. 2017 Aug;281:162-171. doi: 10.1016/j.jmr.2017.06.004. J Magn Reson. 2017. PMID: 28618387
-
Four pulse recoupling.J Magn Reson. 2016 Nov;272:158-165. doi: 10.1016/j.jmr.2016.09.019. Epub 2016 Sep 26. J Magn Reson. 2016. PMID: 27701032
-
Recoupling pulse sequences with constant phase increments.J Magn Reson. 2016 Oct;271:75-82. doi: 10.1016/j.jmr.2016.08.011. Epub 2016 Aug 23. J Magn Reson. 2016. PMID: 27569693
-
Homonuclear dipolar recoupling techniques for structure determination in uniformly 13C-labeled proteins.Solid State Nucl Magn Reson. 2009 Nov;36(3):119-28. doi: 10.1016/j.ssnmr.2009.07.003. Epub 2009 Aug 5. Solid State Nucl Magn Reson. 2009. PMID: 19729285 Review.
Cited by
-
Optimization of band-selective homonuclear dipolar recoupling in solid-state NMR by a numerical phase search.J Chem Phys. 2019 Apr 21;150(15):154201. doi: 10.1063/1.5092986. J Chem Phys. 2019. PMID: 31005077 Free PMC article.
-
Simplified Preservation of Equivalent Pathways Spectroscopy.JACS Au. 2023 Oct 11;3(10):2763-2771. doi: 10.1021/jacsau.3c00312. eCollection 2023 Oct 23. JACS Au. 2023. PMID: 37885577 Free PMC article.
References
-
- Opella SJ. NMR and membrane proteins. Nat Struct Biol. 1997;4:845–848. - PubMed
-
- Griffin RG. Dipolar recoupling in MAS spectra of biological solids. Nat Struct Biol. 1998;5:508–512. - PubMed
-
- Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H. Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature. 2002;420:98–102. - PubMed
Further reading
-
- Palmer AG, III, Cavanagh J, Wright PE, Rance M. Sensitivity improvement in proton- detected 2-dimensional heteronuclear correlation NMR spectroscopy. J Magn Reson. 1991;93:151–170.
-
- Kay LE. Pulsed-field gradient-enhanced three-dimensional NMR experiment for correlating 13Cα/β, 13C′, and 1Hα chemical shifts in uniformly 13C-labeled proteins dissolved in H2O. J Am Chem Soc. 1993;115:2055.
-
- Sattler M, Schmidt P, Schleucher J, Schedletzky O, Glaser SJ, Griesinger C. Novel pulse sequences with sensitivity enhancement for in-phase coherence transfer employing pulsed field gradients. J Magn Reson B. 1995;108:235–242.
-
- Schleucher J, Schwendinger M, Sattler M, Schmidt P, Schedletzky O, Glaser SJ, Sørensen OW, Griesinger C. A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed field gradients. J Biomol NMR. 1994;4:30. - PubMed
-
- Tycko R. Sensitivity enhancement in two-dimensional solid state NMR spectroscopy by transverse mixing. ChemPhysChem. 2004;5:863–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

