Single-cell, time-resolved study of the effects of the antimicrobial peptide alamethicin on Bacillus subtilis
- PMID: 26777771
- PMCID: PMC4779711
- DOI: 10.1016/j.bbamem.2016.01.003
Single-cell, time-resolved study of the effects of the antimicrobial peptide alamethicin on Bacillus subtilis
Abstract
Alamethicin is a well-studied antimicrobial peptide (AMP) that kills Gram-positive bacteria. It forms narrow, barrel-stave pores in planar lipid bilayers. We present a detailed, time-resolved microscopy study of the sequence of events during the attack of alamethicin on individual, live Bacillus subtilis cells expressing GFP in the cytoplasm. At the minimum inhibitory concentration (MIC), the first observed symptom is the halting of growth, as judged by the plateau in measured cell length vs time. The data strongly suggest that this growth-halting event occurs prior to membrane permeabilization. Gradual degradation of the proton-motive force, inferred from a decrease in pH-dependent GFP fluorescence intensity, evidently begins minutes later and continues over about 5 min. There follows an abrupt permeabilization of the cytoplasmic membrane to the DNA stain Sytox Orange and concomitant loss of small osmolytes, causing observable cell shrinkage, presumably due to decreased turgor pressure. This permeabilization of the cytoplasmic membrane occurs uniformly across the entire membrane, not locally, on a timescale of 5s or less. GFP gradually leaks out of the cell envelope, evidently impeded by the shrunken peptidoglycan layer. Disruption of the cell envelope by alamethicin occurs in stages, with larger and larger species permeating the envelope as time evolves over tens of minutes. Some of the observed symptoms are consistent with the formation of barrel-stave pores, but the data do not rule out "chaotic pore" or "carpet" mechanisms. We contrast the effects of alamethicin and the human cathelicidin LL-37 on B. subtilis.
Keywords: Alamethicin; Antimicrobial peptide; Bacillus subtilis; Barrel-stave pore; Fluorescence microscopy; Membrane permeabilization; Real-time imaging; Single-cell imaging.
Copyright © 2016 Elsevier B.V. All rights reserved
Figures
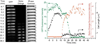

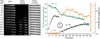



References
-
- Leitgeb B, Szekeres A, Manczinger L, Vagvolgyi C, Kredics L. The history of alamethicin: a review of the most extensively studied peptaibol. Chem Biodivers. 2007;4:1027–1051. - PubMed
-
- Kredics L, Szekeres A, Czifra D, Vagvolgyi C, Leitgeb B. Recent results in alamethicin research. Chem Biodivers. 2013;10:744–771. - PubMed
-
- Stella L, Burattini M, Mazzuca C, Palleschi A, Venanzi M, Coin I, Peggion C, Toniolo C, Pispisa B. Alamethicin interaction with lipid membranes: a spectroscopic study on synthetic analogues. Chem Biodivers. 2007;4:1299–1312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

