Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center
- PMID: 26777991
- PMCID: PMC4767587
- DOI: 10.1016/j.ygyno.2016.01.008
Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center
Abstract
Objective: The aim of this study was to evaluate the use of neoadjuvant chemotherapy (NACT) and primary debulking surgery (PDS) before and after results from a randomized trial were published and showed non-inferiority between NACT and PDS in the management of advanced-stage ovarian carcinoma.
Methods: We evaluated consecutive patients with advanced-stage ovarian cancer treated at our institution from 1/1/08-5/1/13, which encompassed 32 months before and 32 months after the randomized trial results were published. We included all newly diagnosed patients with high-grade histology and stage III/IV disease. Associations between the use of NACT and clinical variables over time were evaluated.
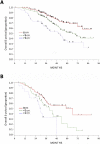
Results: Our study included 586 patients. Median age was 62 years (range, 30-90); 406 patients (69%) had stage III disease, and 570 (97%) had disease of serous histology. Twenty-six percent (154/586) were treated with NACT and 74% (432/586) with PDS. NACT use increased significantly from 22% (56/256) before 2010 (at which point the results of the randomized trial were published) to 30% (98/330) after 2010 (p=0.037). Although patients who underwent PDS were more likely to experience grade 3/4 surgical complications than those who underwent NACT, those selected for PDS had a median OS of 71.7 months (CI, 59.8-not reached) compared with 42.9 months (CI 37.1-56.3) for those selected for NACT.
Conclusions: In this single-institution analysis, the best survival outcomes were observed in patients who were deemed eligible for PDS followed by platinum-based chemotherapy. Selection criteria for NACT require further definition and should take institutional surgical strategy into account.
Keywords: Advanced-stage ovarian cancer; Interval debulking surgery; Neoadjuvant chemotherapy; Overall survival; Primary debulking surgery; Progression-free survival.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
References
-
- American Cancer Society . Cancer Facts & Figures. American cancer society; Atlanta: 2015. [April 24, 2015]. http://www.cancer.org/acs/groups/content/@editorial/documents/document/a... 2015.
-
- [April 24, 2015];Final Recommendation Statement: Ovarian Cancer: Screening. U.S., Preventive Services Task Force. 2014 Oct; http://www.uspreventiveservicestaskforce.org/Page/Document/Recommendatio....
-
- Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int. J. Gynecol. Cancer. 2011;21:750–755. - PubMed
-
- National Comprehensive Cancer Network [April 24, 2015];Epithelial Ovarian Cancer; Including Fallopian Tube Cancer and Primary Peritoneal Cancer, 2015 Version 1.2015) http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
-
- Vergote IB, De Wever I, Decloedt J, Tjalma W, Van Gramberen M, van Dam P. Neoadjuvant chemotherapy versus primary debulking surgery in advanced ovarian cancer. Semin. Oncol. 2000;27(3 Suppl 7):31–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous