Isolation of highly enriched primary human microglia for functional studies
- PMID: 26778406
- PMCID: PMC4725991
- DOI: 10.1038/srep19371
Isolation of highly enriched primary human microglia for functional studies
Abstract
Microglia, the resident macrophages of the central nervous system play vital roles in brain homeostasis through clearance of pathogenic material. Microglia are also implicated in neurological disorders through uncontrolled activation and inflammatory responses. To date, the vast majority of microglial studies have been performed using rodent models. Human microglia differ from rodent counterparts in several aspects including their response to pharmacological substances and their inflammatory secretions. Such differences highlight the need for studies on primary adult human brain microglia and methods to isolate them are therefore required. Our procedure generates microglial cultures of >95% purity from both biopsy and autopsy human brain tissue using a very simple media-based culture procedure that takes advantage of the adherent properties of these cells. Microglia obtained in this manner can be utilised for research within a week. Isolated microglia demonstrate phagocytic ability and respond to inflammatory stimuli and their purity makes them suitable for numerous other forms of in vitro studies, including secretome and transcriptome analysis. Furthermore, this protocol allows for the simultaneous isolation of neural precursor cells during the microglial isolation procedure. As human brain tissue is such a precious and valuable resource the simultaneous isolation of multiple cell types is highly beneficial.
Figures

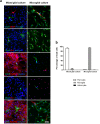


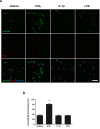
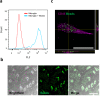

References
-
- Ferguson T. A. & Griffith T. S. A vision of cell death: insights into immune privilege. Immunological reviews 156, 167–184 (1997). - PubMed
-
- del Rio-Hortega P. In Cytology and Cellular Pathology of the Nervous System (ed Penfield W.) Ch. 482–534, (Hoeber, 1932).
-
- Kreutzberg G. W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19, 312–318, 0166-2236(96)10049-7 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

