In vivo, high-frequency three-dimensional cardiac MR elastography: Feasibility in normal volunteers
- PMID: 26778442
- PMCID: PMC4947569
- DOI: 10.1002/mrm.26101
In vivo, high-frequency three-dimensional cardiac MR elastography: Feasibility in normal volunteers
Abstract
Purpose: Noninvasive stiffness imaging techniques (elastography) can image myocardial tissue biomechanics in vivo. For cardiac MR elastography (MRE) techniques, the optimal vibration frequency for in vivo experiments is unknown. Furthermore, the accuracy of cardiac MRE has never been evaluated in a geometrically accurate phantom. Therefore, the purpose of this study was to determine the necessary driving frequency to obtain accurate three-dimensional (3D) cardiac MRE stiffness estimates in a geometrically accurate diastolic cardiac phantom and to determine the optimal vibration frequency that can be introduced in healthy volunteers.
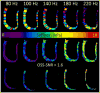
Methods: The 3D cardiac MRE was performed on eight healthy volunteers using 80 Hz, 100 Hz, 140 Hz, 180 Hz, and 220 Hz vibration frequencies. These frequencies were tested in a geometrically accurate diastolic heart phantom and compared with dynamic mechanical analysis (DMA).
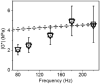
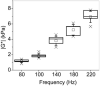
Results: The 3D Cardiac MRE was shown to be feasible in volunteers at frequencies as high as 180 Hz. MRE and DMA agreed within 5% at frequencies greater than 180 Hz in the cardiac phantom. However, octahedral shear strain signal to noise ratios and myocardial coverage was shown to be highest at a frequency of 140 Hz across all subjects.
Conclusion: This study motivates future evaluation of high-frequency 3D MRE in patient populations. Magn Reson Med 77:351-360, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: cardiac MRE; cardiac elastography; myocardial stiffness.
© 2016 Wiley Periodicals, Inc.
Figures








References
-
- Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. - PubMed
-
- Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Ann Rev Biomed Eng. 2005;7:223–253. - PubMed
-
- Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–H512. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

