Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing
- PMID: 26778829
- PMCID: PMC4766044
- DOI: 10.1039/c5lc01396h
Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing
Abstract
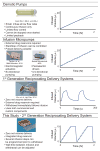
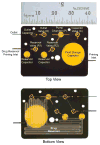
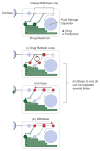
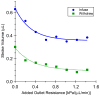
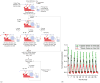
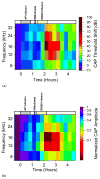
The anatomical and pharmacological inaccessibility of the inner ear is a major challenge in drug-based treatment of auditory disorders. This also makes pharmacokinetic characterization of new drugs with systemic delivery challenging, because efficacy is coupled with how efficiently a drug can reach its target. Direct delivery of drugs to cochlear fluids bypasses pharmacokinetic barriers and helps to minimize systemic toxicity, but anatomical barriers make administration of multiple doses difficult without an automated delivery system. Such a system may be required for hair-cell regeneration treatments, which will likely require timed delivery of several drugs. To address these challenges, we have developed a micropump for controlled, automated inner-ear drug delivery with the ultimate goal of producing a long-term implantable/wearable delivery system. The current pump is designed to be used with a head mount for guinea pigs in preclinical drug characterization experiments. In this system, we have addressed several microfluidic challenges, including maintaining controlled delivery at safe, low flow rates and delivering drug without increasing the volume of fluid in the cochlea. By integrating a drug reservoir and all fluidic components into the microfluidic structure of the pump, we have made the drug delivery system robust compared to previous systems that utilized separate, tubing-connected components. In this study, we characterized the pump's unique infuse-withdraw and on-demand dosing capabilities on the bench and in guinea pig animal models. For the animal experiments, we used DNQX, a glutamate receptor antagonist, as a physiological indicator of drug delivery. DNQX suppresses compound action potentials (CAPs), so we were able to infer the distribution and spreading of the DNQX over time by measuring the changes in CAPs in response to stimuli at several characteristic frequencies.
Figures



Similar articles
-
Microfabricated infuse-withdraw micropump component for an integrated inner-ear drug-delivery platform.Biomed Microdevices. 2015 Apr;17(2):37. doi: 10.1007/s10544-014-9923-8. Biomed Microdevices. 2015. PMID: 25686902 Free PMC article.
-
A microfluidic reciprocating intracochlear drug delivery system with reservoir and active dose control.Lab Chip. 2014 Feb 21;14(4):710-21. doi: 10.1039/c3lc51105g. Lab Chip. 2014. PMID: 24302432 Free PMC article.
-
Development of a microfluidics-based intracochlear drug delivery device.Audiol Neurootol. 2009;14(6):411-22. doi: 10.1159/000241898. Epub 2009 Nov 16. Audiol Neurootol. 2009. PMID: 19923811 Free PMC article.
-
Intracochlear drug delivery systems.Expert Opin Drug Deliv. 2011 Sep;8(9):1161-74. doi: 10.1517/17425247.2011.588207. Epub 2011 May 26. Expert Opin Drug Deliv. 2011. PMID: 21615213 Free PMC article. Review.
-
The role of intracochlear drug delivery devices in the management of inner ear disease.Expert Opin Drug Deliv. 2015 Mar;12(3):465-79. doi: 10.1517/17425247.2015.974548. Epub 2014 Oct 27. Expert Opin Drug Deliv. 2015. PMID: 25347140 Review.
Cited by
-
A Wirelessly Controlled Scalable 3D-Printed Microsystem for Drug Delivery.Pharmaceuticals (Basel). 2021 Jun 4;14(6):538. doi: 10.3390/ph14060538. Pharmaceuticals (Basel). 2021. PMID: 34199855 Free PMC article.
-
Bioenergy-Based Closed-Loop Medical Systems for the Integration of Treatment, Monitoring, and Feedback.Small Sci. 2023 Aug 22;3(10):2300043. doi: 10.1002/smsc.202300043. eCollection 2023 Oct. Small Sci. 2023. PMID: 40213131 Free PMC article.
-
Biomaterial-based drug delivery systems in the treatment of inner ear disorders.J Nanobiotechnology. 2025 Apr 17;23(1):297. doi: 10.1186/s12951-025-03368-0. J Nanobiotechnology. 2025. PMID: 40247337 Free PMC article. Review.
-
Closed-loop feedback control for microfluidic systems through automated capacitive fluid height sensing.Lab Chip. 2018 Mar 13;18(6):902-914. doi: 10.1039/c7lc01223c. Lab Chip. 2018. PMID: 29437172 Free PMC article.
-
Rational Design of Inner Ear Drug Delivery Systems.Adv Sci (Weinh). 2025 Aug;12(29):e2410568. doi: 10.1002/advs.202410568. Epub 2025 May 8. Adv Sci (Weinh). 2025. PMID: 40344640 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

