Understanding How the Subcommissural Organ and Other Periventricular Secretory Structures Contribute via the Cerebrospinal Fluid to Neurogenesis
- PMID: 26778959
- PMCID: PMC4689152
- DOI: 10.3389/fncel.2015.00480
Understanding How the Subcommissural Organ and Other Periventricular Secretory Structures Contribute via the Cerebrospinal Fluid to Neurogenesis
Abstract
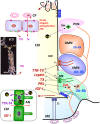
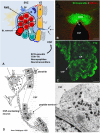
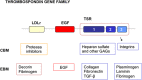
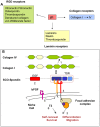
The dynamic and molecular composition of the cerebrospinal fluid (CSF) and, consequently, the CSF physiology is much more complex and fascinating than the simplistic view held for decades. Signal molecules either transported from blood to CSF or secreted into the CSF by circumventricular organs and CSF-contacting neurons, use the CSF to reach their targets in the brain, including the pre- and postnatal neurogenic niche. The subcommissural organ (SCO), a highly conserved brain gland present throughout the vertebrate phylum, is one of the sources for signals, as well as the choroid plexus, tanycytes and CSF-contacting neurons. The SCO secretes into the fetal and adult CSF SCO-spondin, transthyretin, and basic fibroblast growth factor. These proteins participate in certain aspects of neurogenesis, such as cell cycle of neural stem cells, neuronal differentiation, and axon pathfinding. Through the CSF, the SCO-secretory proteins may reach virtually any target in the embryonic and adult central nervous system. Since the SCO continues to secrete throughout life span, it seems likely that the neurogenetic property of the SCO compounds would be targeted to the niches where neurogenesis continues in adulthood. This review is aimed to bring into discussion early and new evidence concerning the role(s) of the SCO, and the probable mechanisms by which SCO compounds can readily reach the neurogenic niche of the subventricular zone flowing with the CSF to participate in the regulation of the neurogenic niche. As we unfold the multiples trans-fluid talks between discrete brain domains we will have more tools to influence such talks.
Keywords: CSF-contacting neurons; SCO-spondin; cerebrospinal fluid; circumventricular organs; integrins; neurogenesis; subcommissural organ; transthyretin.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

