β-Secretase 1's Targeting Reduces Hyperphosphorilated Tau, Implying Autophagy Actors in 3xTg-AD Mice
- PMID: 26778963
- PMCID: PMC4705306
- DOI: 10.3389/fncel.2015.00498
β-Secretase 1's Targeting Reduces Hyperphosphorilated Tau, Implying Autophagy Actors in 3xTg-AD Mice
Abstract
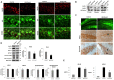
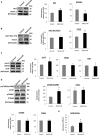
β-site APP cleaving enzyme 1 (BACE1) initiates APP cleavage, which has been reported to be an inducer of tau pathology by altering proteasome functions in Alzheimer's disease (AD). However, the exact relationship between BACE1 and PHF (Paired Helical Filaments) formation is not clear. In this study, we confirm that BACE1 and Hsc70 are upregulated in the brains of AD patients, and we demonstrate that both proteins show enhanced expression in lipid rafts from AD-affected triple transgenic mouse brains. BACE1 targeting increased Hsc70 levels in the membrane and cytoplasm fractions and downregulated Hsp90 and CHIP in the nucleus in the hippocampi of 3xTg-AD mice. However, these observations occurred in a proteasome-independent manner in vitro. The BACE1miR-induced reduction of soluble hyperphosphorylated tau was associated with a decrease in MAPK activity. However, the BACE1 RNAi-mediated reduction of hyperphosphorylated tau was only blocked by 3-MA (3-methyladenine) in vitro, and it resulted in the increase of Hsc70 and LAMP2 in lipid rafts from hippocampi of 3xTg-AD mice, and upregulation of survival and homeostasis signaling. In summary, our findings suggest that BACE1 silencing neuroprotects reducing soluble hyperphosphorylated tau, modulating certain autophagy-related proteins in aged 3xTg-AD mice.
Keywords: Alzheimer’s disease; autophagy; chaperones; lipid rafts; tauopathy; β-secretase 1.
Figures






Similar articles
-
Selenomethionine Mitigates Cognitive Decline by Targeting Both Tau Hyperphosphorylation and Autophagic Clearance in an Alzheimer's Disease Mouse Model.J Neurosci. 2017 Mar 1;37(9):2449-2462. doi: 10.1523/JNEUROSCI.3229-16.2017. Epub 2017 Jan 30. J Neurosci. 2017. PMID: 28137967 Free PMC article.
-
Toluidine blue O modifies hippocampal amyloid pathology in a transgenic mouse model of Alzheimer's disease.Biochimie. 2018 Mar;146:105-112. doi: 10.1016/j.biochi.2017.12.004. Epub 2017 Dec 14. Biochimie. 2018. PMID: 29248542
-
Relationship between ubiquilin-1 and BACE1 in human Alzheimer's disease and APdE9 transgenic mouse brain and cell-based models.Neurobiol Dis. 2016 Jan;85:187-205. doi: 10.1016/j.nbd.2015.11.005. Epub 2015 Nov 10. Neurobiol Dis. 2016. PMID: 26563932
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
-
BACE1 RNAi Restores the Composition of Phosphatidylethanolamine-Derivates Related to Memory Improvement in Aged 3xTg-AD Mice.Front Cell Neurosci. 2016 Nov 11;10:260. doi: 10.3389/fncel.2016.00260. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27891075 Free PMC article.
-
Bioinformatic analysis of hippocampal histopathology in Alzheimer's disease and the therapeutic effects of active components of traditional Chinese medicine.Front Pharmacol. 2024 Aug 16;15:1424803. doi: 10.3389/fphar.2024.1424803. eCollection 2024. Front Pharmacol. 2024. PMID: 39221152 Free PMC article.
-
Neurodegeneration and convergent factors contributing to the deterioration of the cytoskeleton in Alzheimer's disease, cerebral ischemia and multiple sclerosis (Review).Biomed Rep. 2022 Apr;16(4):27. doi: 10.3892/br.2022.1510. Epub 2022 Feb 18. Biomed Rep. 2022. PMID: 35251614 Free PMC article. Review.
-
Diosgenin restores Aβ-induced axonal degeneration by reducing the expression of heat shock cognate 70 (HSC70).Sci Rep. 2018 Aug 3;8(1):11707. doi: 10.1038/s41598-018-30102-8. Sci Rep. 2018. PMID: 30076345 Free PMC article.
-
Beta-Secretase 1 Underlies Reactive Astrocytes and Endothelial Disruption in Neurodegeneration.Front Cell Neurosci. 2021 May 6;15:656832. doi: 10.3389/fncel.2021.656832. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34025357 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

