A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls
- PMID: 26778976
- PMCID: PMC4705224
- DOI: 10.3389/fnsys.2015.00175
A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls
Abstract
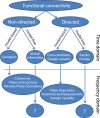
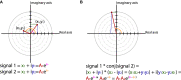
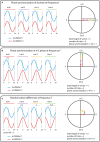
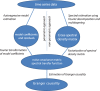
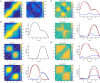
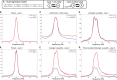
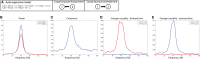
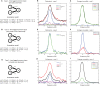
Oscillatory neuronal activity may provide a mechanism for dynamic network coordination. Rhythmic neuronal interactions can be quantified using multiple metrics, each with their own advantages and disadvantages. This tutorial will review and summarize current analysis methods used in the field of invasive and non-invasive electrophysiology to study the dynamic connections between neuronal populations. First, we review metrics for functional connectivity, including coherence, phase synchronization, phase-slope index, and Granger causality, with the specific aim to provide an intuition for how these metrics work, as well as their quantitative definition. Next, we highlight a number of interpretational caveats and common pitfalls that can arise when performing functional connectivity analysis, including the common reference problem, the signal to noise ratio problem, the volume conduction problem, the common input problem, and the sample size bias problem. These pitfalls will be illustrated by presenting a set of MATLAB-scripts, which can be executed by the reader to simulate each of these potential problems. We discuss how these issues can be addressed using current methods.
Keywords: coherence analysis; electrophysiology; functional connectivity (FC); granger causality; oscillations; phase synchronization.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

