Role of Prefrontal Persistent Activity in Working Memory
- PMID: 26778980
- PMCID: PMC4700146
- DOI: 10.3389/fnsys.2015.00181
Role of Prefrontal Persistent Activity in Working Memory
Abstract
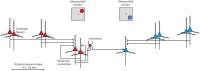
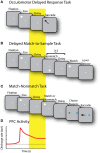
The prefrontal cortex is activated during working memory, as evidenced by fMRI results in human studies and neurophysiological recordings in animal models. Persistent activity during the delay period of working memory tasks, after the offset of stimuli that subjects are required to remember, has traditionally been thought of as the neural correlate of working memory. In the last few years several findings have cast doubt on the role of this activity. By some accounts, activity in other brain areas, such as the primary visual and posterior parietal cortex, is a better predictor of information maintained in visual working memory and working memory performance; dynamic patterns of activity may convey information without requiring persistent activity at all; and prefrontal neurons may be ill-suited to represent non-spatial information about the features and identity of remembered stimuli. Alternative interpretations about the role of the prefrontal cortex have thus been suggested, such as that it provides a top-down control of information represented in other brain areas, rather than maintaining a working memory trace itself. Here we review evidence for and against the role of prefrontal persistent activity, with a focus on visual neurophysiology. We show that persistent activity predicts behavioral parameters precisely in working memory tasks. We illustrate that prefrontal cortex represents features of stimuli other than their spatial location, and that this information is largely absent from early cortical areas during working memory. We examine memory models not dependent on persistent activity, and conclude that each of those models could mediate only a limited range of memory-dependent behaviors. We review activity decoded from brain areas other than the prefrontal cortex during working memory and demonstrate that these areas alone cannot mediate working memory maintenance, particularly in the presence of distractors. We finally discuss the discrepancy between BOLD activation and spiking activity findings, and point out that fMRI methods do not currently have the spatial resolution necessary to decode information within the prefrontal cortex, which is likely organized at the micrometer scale. Therefore, we make the case that prefrontal persistent activity is both necessary and sufficient for the maintenance of information in working memory.
Keywords: fMRI; monkey; neuron; neurophysiology; prefrontal cortex.
Figures


References
-
- Atkinson R. C., Shiffrin R. M. (1968). Human memory: a proposed system and its control processes, in The Psychology of Learning and Motivation, eds Spence K. W., Spence J. T. (London: Academic Press; ), 89–195.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

