Acute Myocardial Response to Stretch: What We (don't) Know
- PMID: 26779036
- PMCID: PMC4700209
- DOI: 10.3389/fphys.2015.00408
Acute Myocardial Response to Stretch: What We (don't) Know
Abstract
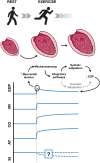
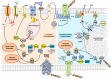
Myocardial stretch, as result of acute hemodynamic overload, is one of the most frequent challenges to the heart and the ability of the heart to intrinsically adapt to it is essential to prevent circulatory congestion. In this review, we highlight the historical background, the currently known mechanisms, as well as the gaps in the understanding of this physiological response. The systolic adaptation to stretch is well-known for over 100 years, being dependent on an immediate increase in contractility-known as the Frank-Starling mechanism-and a further progressive increase-the slow force response. On the other hand, its diastolic counterpart remains largely unstudied. Mechanosensors are structures capable of perceiving mechanical signals and activating pathways that allow their transduction into biochemical responses. Although the connection between these structures and stretch activated pathways remains elusive, we emphasize those most likely responsible for the initiation of the acute response. Calcium-dependent pathways, including angiotensin- and endothelin-related pathways; and cGMP-dependent pathways, comprising the effects of nitric oxide and cardiac natriuretic hormones, embody downstream signaling. The ischemic setting, a paradigmatic situation of acute hemodynamic overload, is also touched upon. Despite the relevant knowledge accumulated, there is much that we still do not know. The quest for further understanding the myocardial response to acute stretch may provide new insights, not only in its physiological importance, but also in the prevention and treatment of cardiovascular diseases.
Keywords: cardiac function; frank starling mechanism; myocardial stretch; neurohumoral adaptation; slow force response.
Figures

Comment in
-
Commentary: Acute Myocardial Response to Stretch: What We (don't) Know.Front Physiol. 2017 Mar 2;8:121. doi: 10.3389/fphys.2017.00121. eCollection 2017. Front Physiol. 2017. PMID: 28303106 Free PMC article. No abstract available.
References
-
- Alvarez B. V., Pérez N. G., Ennis I. L., Camilión De Hurtado M. C., Cingolani H. E. (1999). Mechanisms underlying the increase in force and Ca2+ transient that follow stretch of cardiac muscle: a possible explanation of the anrep effect. Circ. Res. 85, 716–722. 10.1161/01.RES.85.8.716 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

