Application of a Scalable Plant Transient Gene Expression Platform for Malaria Vaccine Development
- PMID: 26779197
- PMCID: PMC4688378
- DOI: 10.3389/fpls.2015.01169
Application of a Scalable Plant Transient Gene Expression Platform for Malaria Vaccine Development
Abstract
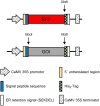
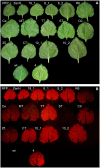
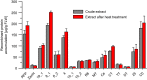
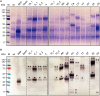
Despite decades of intensive research efforts there is currently no vaccine that provides sustained sterile immunity against malaria. In this context, a large number of targets from the different stages of the Plasmodium falciparum life cycle have been evaluated as vaccine candidates. None of these candidates has fulfilled expectations, and as long as we lack a single target that induces strain-transcending protective immune responses, combining key antigens from different life cycle stages seems to be the most promising route toward the development of efficacious malaria vaccines. After the identification of potential targets using approaches such as omics-based technology and reverse immunology, the rapid expression, purification, and characterization of these proteins, as well as the generation and analysis of fusion constructs combining different promising antigens or antigen domains before committing to expensive and time consuming clinical development, represents one of the bottlenecks in the vaccine development pipeline. The production of recombinant proteins by transient gene expression in plants is a robust and versatile alternative to cell-based microbial and eukaryotic production platforms. The transfection of plant tissues and/or whole plants using Agrobacterium tumefaciens offers a low technical entry barrier, low costs, and a high degree of flexibility embedded within a rapid and scalable workflow. Recombinant proteins can easily be targeted to different subcellular compartments according to their physicochemical requirements, including post-translational modifications, to ensure optimal yields of high quality product, and to support simple and economical downstream processing. Here, we demonstrate the use of a plant transient expression platform based on transfection with A. tumefaciens as essential component of a malaria vaccine development workflow involving screens for expression, solubility, and stability using fluorescent fusion proteins. Our results have been implemented for the evidence-based iterative design and expression of vaccine candidates combining suitable P. falciparum antigen domains. The antigens were also produced, purified, and characterized in further studies by taking advantage of the scalability of this platform.
Keywords: Nicotiana benthamiana plants; Plasmodium falciparum; expression screening; heat stability; multi domain-fusion antigens; red fluorescent protein.
Figures

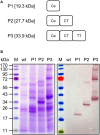
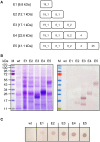


References
-
- Beiss V., Spiegel H., Boes A., Kapelski S., Scheuermayer M., Edgue G., et al. . (2015). Heat-precipitation allows the efficient purification of a functional plant-derived malaria transmission-blocking vaccine candidate fusion protein. Biotechnol. Bioeng. 112, 1297–1305. 10.1002/bit.25548 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

