The effect of cell disruption techniques and chaotropic agents on the downstream purification process of mecasermin produced as inclusion body in E. coli
- PMID: 26779275
- PMCID: PMC4698866
The effect of cell disruption techniques and chaotropic agents on the downstream purification process of mecasermin produced as inclusion body in E. coli
Abstract
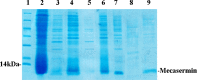
The isolation of the target protein from inclusion bodies (IBs) is a preliminary step to increase protein titer and to maintain its biological activity. In the present study, the effects of various cell lysis methods and the expression temperature was investigated on the improvement of the subsequent purification steps of mecasermin produced in IB. We also investigated the solubilization profile of the top-notch IB in 6 M guanidine hydrochloride (Gdn-HCl) and 8 M urea at different pH ranges. Mecasermin was expressed at various temperatures (25, 28, 30, and 37 °C) and the Escherichia coli cells were lysed by different cell lysis methods. The purity and quality of harvested IBs was evaluated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Finally, mecasermin was refolded and purified using gel filtration chromatography. The profile of SDS-PAGE analysis showed higher quality and purity after application of sonication coupled with lysozyme pretreatment for expressed mecasermin at 37 °C. Besides, from dithiothreitol application in washing step, we achieved a manifold enriched secondary IB for further purification of mecasermin. Mecasermin exhibited optimized solubility in 6 M Gdn-HCl at pH of 5.4. The findings of this study indicate an important role for cell disruption techniques to efficient purification of mecasermin. The study presents the most efficient techniques for improvement of downstream purification of mecasermin.
Keywords: Cell disruption; Inclusion body purification; Mecasermin; Strong chaotrope.
Figures



Similar articles
-
Efficient process development for high-level production, purification, formulation, and characterization of recombinant mecasermin in Escherichia coli.Biotechnol Appl Biochem. 2021 Aug;68(4):776-788. doi: 10.1002/bab.1990. Epub 2020 Aug 7. Biotechnol Appl Biochem. 2021. PMID: 32692415
-
Recombinant production of mecasermin in E. coli expression system.Res Pharm Sci. 2014 Nov-Dec;9(6):453-61. Res Pharm Sci. 2014. PMID: 26339260 Free PMC article.
-
Refolding and purification of recombinant L-asparaginase from inclusion bodies of E. coli into active tetrameric protein.Front Microbiol. 2014 Sep 15;5:486. doi: 10.3389/fmicb.2014.00486. eCollection 2014. Front Microbiol. 2014. PMID: 25309524 Free PMC article.
-
Mecasermin rinfabate: insulin-like growth factor-I/insulin-like growth factor binding protein-3, mecaserimin rinfibate, rhIGF-I/rhIGFBP-3.Drugs R D. 2005;6(2):120-7. doi: 10.2165/00126839-200506020-00008. Drugs R D. 2005. PMID: 15777106 Review.
-
Mecasermin for primary insulin-like growth factor-1 deficiency.Aust Prescr. 2022 Dec;45(6):215. doi: 10.18773/austprescr.2022.077. Epub 2022 Dec 1. Aust Prescr. 2022. PMID: 36479333 Free PMC article. Review. No abstract available.
Cited by
-
Purification of Soluble Membrane-Bound Ambystoma mexicanum Epidermal Lipoxygenase from E. coli and Its Growth Effect on Human Fetal Foreskin Fibroblast.Protein J. 2020 Aug;39(4):377-382. doi: 10.1007/s10930-020-09898-w. Protein J. 2020. PMID: 32285244
References
-
- Rosenbloom AL. Mecasermin (Recombinant Human Insulin-like Growth Factor I) Adv Ther. 2009;26:40–54. - PubMed
-
- A Sato S, Koyama H, Yamada S, Suzuki KI, Tamura K, Kobayashi M, et al. Three-dimensional solution structure of a disulfide bond isomer of the human insulin-like growth factor-I. J Pept Res. 2000;56:218–230. - PubMed
-
- Kumar Panda A. Bioprocessing of therapeutic proteins from the inclusion bodies of Escherichia coli. Adv Biochem Eng Biotechnol. 2003;85:43–93. - PubMed
-
- Frederick M. 4th ed. Vol. 2. New York: Wiley; Ausubel. Current Protocols in Molecular Biology; pp. 1252–1256.
LinkOut - more resources
Full Text Sources
