Pedunculopontine arousal system physiology - Deep brain stimulation (DBS)
- PMID: 26779322
- PMCID: PMC4688589
- DOI: 10.1016/j.slsci.2015.09.001
Pedunculopontine arousal system physiology - Deep brain stimulation (DBS)
Abstract
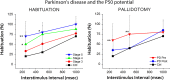
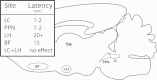
This review describes the wake/sleep symptoms present in Parkinson׳s disease, and the role of the pedunculopontine nucleus in these symptoms. The physiology of PPN cells is important not only because it is a major element of the reticular activating system, but also because it is a novel target for deep brain stimulation in the treatment of gait and postural deficits in Parkinson׳s disease. A greater understanding of the physiology of the target nuclei within the brainstem and basal ganglia, amassed over the past decades, has enabled increasingly better patient outcomes from deep brain stimulation for movement disorders.
Keywords: Basal forebrain; Calcium channels; DBS, deep brain stimulation; EEG, electroencephalogram; Gamma band activity; LC, locus coeruleus; Lateral hypothalamus; Orexin; PD, Parkinson׳s disease; PGO, ponto-geniculo-occipital; PPN, pedunculopontine nucleus; RAS, reticular activating system; REM, rapid eye movement; SN, substantia nigra; STN, subthalamic nucleus; SubCD, subcoeruleus nucleus dorsalis; Tuberomammillary.
Figures

References
-
- Jellinger K.A. Pathology of Parkinson׳s disease: changes other than the nigrostriatal pathway. Mol Med Neuropathol. 1991;14:153–197. - PubMed
-
- Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson׳s disease. Neurobiol Aging. 2003;24:197–211. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

