Bridging academic science and clinical research in the search for novel targeted anti-cancer agents
- PMID: 26779369
- PMCID: PMC4706519
- DOI: 10.7497/j.issn.2095-3941.2015.0079
Bridging academic science and clinical research in the search for novel targeted anti-cancer agents
Abstract
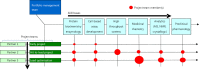
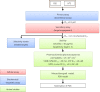
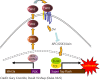
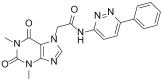
This review starts with a brief history of drug discovery & development, and the place of Asia in this worldwide effort discussed. The conditions and constraints of a successful translational R&D involving academic basic research and clinical research are discussed and the Singapore model for pursuit of open R&D described. The importance of well-characterized, validated drug targets for the search for novel targeted anti-cancer agents is emphasized, as well as a structured, high quality translational R&D. Furthermore, the characteristics of an attractive preclinical development drug candidate are discussed laying the foundation of a successful preclinical development. The most frequent sources of failures are described and risk management at every stage is highly recommended. Organizational factors are also considered to play an important role. The factors to consider before starting a new drug discovery & development project are described, and an example is given of a successful clinical project that has had its roots in local universities and was carried through preclinical development into phase I clinical trials.
Keywords: Drug discovery; drug development; targeted anti-cancer agents; translational R&D.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures

Similar articles
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Translational science: past, present, and future.Biotechniques. 2008 Feb;44(2):ii-viii. doi: 10.2144/000112749. Biotechniques. 2008. PMID: 18422489 Review.
-
Cancer Drug Development: New Targets for Cancer Treatment.Oncologist. 1996;1(3):II-III. Oncologist. 1996. PMID: 10387987
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
-
Editorial: Current status and perspective on drug targets in tubercle bacilli and drug design of antituberculous agents based on structure-activity relationship.Curr Pharm Des. 2014;20(27):4305-6. doi: 10.2174/1381612819666131118203915. Curr Pharm Des. 2014. PMID: 24245755
Cited by
-
The Community Oncology and Academic Medical Center Alliance in the Age of Precision Medicine: Cancer Genetics and Genomics Considerations.J Clin Med. 2020 Jul 6;9(7):2125. doi: 10.3390/jcm9072125. J Clin Med. 2020. PMID: 32640668 Free PMC article. Review.
-
[Cutaneous side effects of targeted cancer drugs].Hautarzt. 2017 Jan;68(1):12-18. doi: 10.1007/s00105-016-3902-3. Hautarzt. 2017. PMID: 27885401 Review. German.
References
-
- Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov 2011;10:428-438. - PubMed
-
- Owens PK, Raddad E, Miller JW, Stille JR, Olovich KG, Smith NV, et al. A decade of innovation in pharmaceutical R&D: the Chorus model. Nat Rev Drug Discov 2015;14:17-28. - PubMed
-
- Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 2014;13:419-431. - PubMed
-
- LaMattina JL. The impact of mergers on pharmaceutical R&D. Nat Rev Drug Discov 2011;10:559-560. - PubMed
-
- Tralau-Stewart C, Low CM, Marlin N. UK academic drug discovery. Nat Rev Drug Discov 2014;13:15-16. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
