Why is start codon selection so precise in eukaryotes?
- PMID: 26779403
- PMCID: PMC4705826
- DOI: 10.4161/trla.28387
Why is start codon selection so precise in eukaryotes?
Abstract
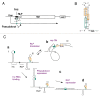
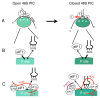
Translation generally initiates with the AUG codon. While initiation at GUG and UUG is permitted in prokaryotes (Archaea and Bacteria), cases of CUG initiation were recently reported in human cells. The varying stringency in translation initiation between eukaryotic and prokaryotic domains largely stems from a fundamental problem for the ribosome in recognizing a codon at the peptidyl-tRNA binding site. Initiation factors specific to each domain of life evolved to confer stringent initiation by the ribosome. The mechanistic basis for high accuracy in eukaryotic initiation is described based on recent findings concerning the role of the multifactor complex (MFC) in this process. Also discussed are whether non-AUG initiation plays any role in translational control and whether start codon accuracy is regulated in eukaryotes.
Keywords: archaeal translation; bacterial translation; eukaryotic translation; initiation factors; multifactor complex; non-AUG initiation; ribosome; start codon fidelity; start codon selection; translation initiation.
Figures


References
-
- Moore PB, Steitz TA.. The involvement of RNA in ribosome function. Nature 2002; 418:229 - 35; http://dx.doi.org/10.1038/418229a; PMID: 12110899 - DOI - PubMed
-
- Ramakrishnan V.. Ribosome structure and the mechanism of translation. Cell 2002; 108:557 - 72; http://dx.doi.org/10.1016/S0092-8674(02)00619-0; PMID: 11909526 - DOI - PubMed
-
- Rodnina MV, Beringer M, Wintermeyer W.. How ribosomes make peptide bonds. Trends Biochem Sci 2007; 32:20 - 6; http://dx.doi.org/10.1016/j.tibs.2006.11.007; PMID: 17157507 - DOI - PubMed
-
- Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FV 4th, Weir JR, Ramakrishnan V.. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 2009; 326:688 - 94; http://dx.doi.org/10.1126/science.1179700; PMID: 19833920 - DOI - PMC - PubMed
-
- Asano K, Ito K. in Encyclopedia of Systems Biology eds Werner Dubitzky, Olaf Wolkenhauser, Kwang-Hyun Cho, & Hiroki Yokota) 2259-2263 (Springer, 2013).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
