Shigella IpaH Family Effectors as a Versatile Model for Studying Pathogenic Bacteria
- PMID: 26779450
- PMCID: PMC4701945
- DOI: 10.3389/fcimb.2015.00100
Shigella IpaH Family Effectors as a Versatile Model for Studying Pathogenic Bacteria
Abstract
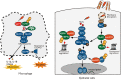
Shigella spp. are highly adapted human pathogens that cause bacillary dysentery (shigellosis). Via the type III secretion system (T3SS), Shigella deliver a subset of virulence proteins (effectors) that are responsible for pathogenesis, with functions including pyroptosis, invasion of the epithelial cells, intracellular survival, and evasion of host immune responses. Intriguingly, T3SS effector activity and strategies are not unique to Shigella, but are shared by many other bacterial pathogens, including Salmonella, Yersinia, and enteropathogenic Escherichia coli (EPEC). Therefore, studying Shigella T3SS effectors will not only improve our understanding of bacterial infection systems, but also provide a molecular basis for developing live bacterial vaccines and antibacterial drugs. One of Shigella T3SS effectors, IpaH family proteins, which have E3 ubiquitin ligase activity and are widely conserved among other bacterial pathogens, are very relevant because they promote bacterial survival by triggering cell death and modulating the host immune responses. Here, we describe selected examples of Shigella pathogenesis, with particular emphasis on the roles of IpaH family effectors, which shed new light on bacterial survival strategies and provide clues about how to overcome bacterial infections.
Keywords: E3 ligase; NF-kB; Shigella; effector; ubiquitin.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

