Exploring cardiac biophysical properties
- PMID: 26779498
- PMCID: PMC4448074
- DOI: 10.5339/gcsp.2015.10
Exploring cardiac biophysical properties
Abstract
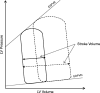
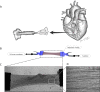
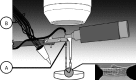
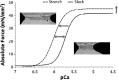
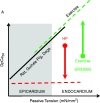
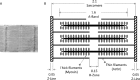
The heart is subject to multiple sources of stress. To maintain its normal function, and successfully overcome these stresses, heart muscle is equipped with fine-tuned regulatory mechanisms. Some of these mechanisms are inherent within the myocardium itself and are known as intrinsic mechanisms. Over a century ago, Otto Frank and Ernest Starling described an intrinsic mechanism by which the heart, even ex vivo, regulates its function on a beat-to-beat basis. According to this phenomenon, the higher the ventricular filling is, the bigger the stroke volume. Thus, the Frank-Starling law establishes a direct relationship between the diastolic and systolic function of the heart. To observe this biophysical phenomenon and to investigate it, technologic development has been a pre-requisite to scientific knowledge. It allowed for example to observe, at the cellular level, a Frank-Starling like mechanism and has been termed: Length Dependent Activation (LDA). In this review, we summarize some experimental systems that have been developed and are currently still in use to investigate cardiac biophysical properties from the whole heart down to the single myofibril. As a scientific support, investigation of the Frank-Starling mechanism will be used as a case study.
Figures


Similar articles
-
Historical perspective on heart function: the Frank-Starling Law.Biophys Rev. 2015 Dec;7(4):421-447. doi: 10.1007/s12551-015-0184-4. Epub 2015 Nov 19. Biophys Rev. 2015. PMID: 28510104 Free PMC article. Review.
-
β-Arrestin mediates the Frank-Starling mechanism of cardiac contractility.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14426-14431. doi: 10.1073/pnas.1609308113. Epub 2016 Nov 28. Proc Natl Acad Sci U S A. 2016. PMID: 27911784 Free PMC article.
-
Frank-Starling mechanism, fluid responsiveness, and length-dependent activation: Unravelling the multiscale behaviors with an in silico analysis.PLoS Comput Biol. 2021 Oct 11;17(10):e1009469. doi: 10.1371/journal.pcbi.1009469. eCollection 2021 Oct. PLoS Comput Biol. 2021. PMID: 34634040 Free PMC article.
-
Re-visiting the Frank-Starling nexus.Prog Biophys Mol Biol. 2021 Jan;159:10-21. doi: 10.1016/j.pbiomolbio.2020.04.003. Epub 2020 May 11. Prog Biophys Mol Biol. 2021. PMID: 32407748 Review.
-
Myocardial response to incremental exercise in endurance-trained athletes: influence of heart rate, contractility and the Frank-Starling effect.Exp Physiol. 2002 Sep;87(5):613-22. doi: 10.1113/eph8702372. Exp Physiol. 2002. PMID: 12481936
Cited by
-
Septic cardiomyopathy: evidence for a reduced force-generating capacity of human atrial myocardium in acute infective endocarditis.Innov Surg Sci. 2017 Apr 13;2(2):81-87. doi: 10.1515/iss-2016-0202. eCollection 2017 Jun. Innov Surg Sci. 2017. PMID: 31579740 Free PMC article.
-
Myocardial Contractility: Historical and Contemporary Considerations.Front Physiol. 2020 Mar 31;11:222. doi: 10.3389/fphys.2020.00222. eCollection 2020. Front Physiol. 2020. PMID: 32296340 Free PMC article.
-
Effects of Normobaric Hypoxia and Adrenergic Blockade over 72 h on Cardiac Function in Rats.Int J Mol Sci. 2023 Jul 13;24(14):11417. doi: 10.3390/ijms241411417. Int J Mol Sci. 2023. PMID: 37511176 Free PMC article.
-
Cardiomyopathies and Related Changes in Contractility of Human Heart Muscle.Int J Mol Sci. 2018 Jul 31;19(8):2234. doi: 10.3390/ijms19082234. Int J Mol Sci. 2018. PMID: 30065175 Free PMC article. Review.
-
TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts.Cell Biosci. 2019 Dec 9;9:98. doi: 10.1186/s13578-019-0362-3. eCollection 2019. Cell Biosci. 2019. PMID: 31827764 Free PMC article. Review.
References
-
- Zimmer H-G. The Isolated Perfused Heart and Its Pioneers. News Physiol Sci. 1998;13:203–210. - PubMed
-
- Sagawa K, Lie RK, Schaefer J. Translation of Otto Frank's paper “Die Grundform des Arteriellen Pulses”. Zeitschrift fur Biologie. 1990;22(3):253–254. - PubMed
-
- Brady AJ. Mechanical properties of isolated cardiac myocytes. Physiol Rev. 1991;71(2):413–428. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
