One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior
- PMID: 26780725
- PMCID: PMC5402896
- DOI: 10.1016/j.matbio.2016.01.004
One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior
Abstract
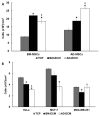
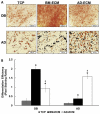
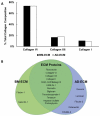
For more than 100years, cells and tissues have been studied in vitro using glass and plastic surfaces. Over the last 10-20years, a great body of research has shown that cells are acutely sensitive to their local environment (extracellular matrix, ECM) which contains both chemical and physical cues that influence cell behavior. These observations suggest that modern cell culture systems, using tissue culture polystyrene (TCP) surfaces, may fail to reproduce authentic cell behavior in vitro, resulting in "artificial outcomes." In the current study, we use bone marrow (BM)- and adipose (AD)-derived stromal cells to prepare BM-ECM and AD-ECM, which are decellularized after synthesis by the cells, to mimic the cellular niche for each of these tissues. Each ECM was characterized for its ability to affect BM- and AD-mesenchymal stem cell (MSC) proliferation, as well as proliferation of three cancer cell lines (HeLa, MCF-7, and MDA-MB-231), modulate cell spreading, and direct differentiation relative to standard TCP surfaces. We found that both ECMs promoted the proliferation of MSCs, but that this effect was enhanced when the tissue-origin of the cells matched that of the ECM (i.e. BM-ECM promoted the proliferation of BM-MSCs over AD-MSCs, and vice versa). Moreover, BM- and AD-ECM were shown to preferentially direct MSC differentiation towards either osteogenic or adipogenic lineage, respectively, suggesting that the effects of the ECM were tissue-specific. Further, each ECM influenced cell morphology (i.e. circularity), irrespective of the origin of the MSCs, lending more support to the idea that effects were tissue specific. Interestingly, unlike MSCs, these ECMs did not promote the proliferation of the cancer cells. In an effort to further understand how these three culture substrates influence cell behavior, we evaluated the chemical (protein composition) and physical properties (architecture and mechanical) of the two ECMs. While many structural proteins (e.g. collagen and fibronectin) were found at equivalent levels in both BM- and AD-ECM, the architecture (i.e. fiber orientation; surface roughness) and physical properties (storage modulus, surface energy) of each were unique. These results, demonstrating differences in cell behavior when cultured on the three different substrates (BM- and AD-ECM and TCP) with differences in chemical and physical properties, provide evidence that the two ECMs may recapitulate specific elements of the native stem cell niche for bone marrow and adipose tissues. More broadly, it could be argued that ECMs, elaborated by cells ex vivo, serve as an ideal starting point for developing tissue-specific culture environments. In contrast to TCP, which relies on the "one size fits all" paradigm, native tissue-specific ECM may be a more rational model to approach engineering 3D tissue-specific culture systems to replicate the in vivo niche. We suggest that this approach will provide more meaningful information for basic research studies of cell behavior as well as cell-based therapeutics.
Keywords: Cell culture; Cell microenvironment; Differentiation; Extracellular matrix; Mesenchymal stem cells (MSCs); Niche.
Published by Elsevier B.V.
Figures




References
-
- Sanford KK, Earle WR. The growth in vitro of single isolated tissue cells. J. Natl. Cancer Inst. 1948;9:229–246. - PubMed
-
- Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. - PubMed
-
- Baker M. Stem cells in culture: defining the substrate. Nat. Methods. 2011;8:293–297.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

