The cerebellum linearly encodes whisker position during voluntary movement
- PMID: 26780828
- PMCID: PMC4737656
- DOI: 10.7554/eLife.10509
The cerebellum linearly encodes whisker position during voluntary movement
Abstract
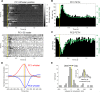
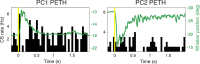
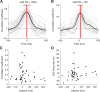
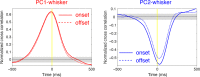
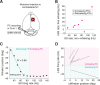
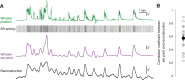
Active whisking is an important model sensorimotor behavior, but the function of the cerebellum in the rodent whisker system is unknown. We have made patch clamp recordings from Purkinje cells in vivo to identify whether cerebellar output encodes kinematic features of whisking including the phase and set point. We show that Purkinje cell spiking activity changes strongly during whisking bouts. On average, the changes in simple spike rate coincide with or slightly precede movement, indicating that the synaptic drive responsible for these changes is predominantly of efferent (motor) rather than re-afferent (sensory) origin. Remarkably, on-going changes in simple spike rate provide an accurate linear read-out of whisker set point. Thus, despite receiving several hundred thousand discrete synaptic inputs across a non-linear dendritic tree, Purkinje cells integrate parallel fiber input to generate precise information about whisking kinematics through linear changes in firing rate.
Keywords: Crus I; Purkinje cell; mouse; neuroscience; simple spike; vibrissa.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





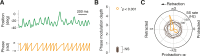
References
-
- Albus JS. A theory of cerebellar function. Mathematical Biosciences. 1971;10:25–61. doi: 10.1016/0025-5564(71)90051-4. - DOI
-
- Bosman LWJ, Houweling AR, Owens CB, Tanke N, Shevchouk OT, Rahmati N, Teunissen WHT, Ju C, Gong W, Koekkoek SKE, De Zeeuw CI. Anatomical pathways involved in generating and sensing rhythmic whisker movements. Frontiers in Integrative Neuroscience. 2011;5 doi: 10.3389/fnint.2011.00053. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

