Reduction of fibroblast size/mechanical force down-regulates TGF-β type II receptor: implications for human skin aging
- PMID: 26780887
- PMCID: PMC4717276
- DOI: 10.1111/acel.12410
Reduction of fibroblast size/mechanical force down-regulates TGF-β type II receptor: implications for human skin aging
Abstract
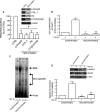
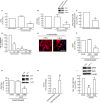
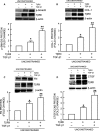
The structural integrity of human skin is largely dependent on the quality of the dermal extracellular matrix (ECM), which is produced, organized, and maintained by dermal fibroblasts. Normally, fibroblasts attach to the ECM and thereby achieve stretched, elongated morphology. A prominent characteristic of dermal fibroblasts in aged skin is reduced size, with decreased elongation and a more rounded, collapsed morphology. Here, we show that reduced size of fibroblasts in mechanically unrestrained three-dimensional collagen lattices coincides with reduced mechanical force, measured by atomic force microscopy. Reduced size/mechanical force specifically down-regulates TGF-β type II receptor (TβRII) and thus impairs TGF-β/Smad signaling pathway. Both TβRII mRNA and protein were decreased, resulting in 90% loss of TGF-β binding to fibroblasts. Down-regulation of TβRII was associated with significantly decreased phosphorylation, DNA-binding, and transcriptional activity of its key downstream effector Smad3 and reduced expression of Smad3-regulated essential ECM components type I collagen, fibronectin, and connective tissue growth factor (CTGF/CCN2). Restoration of TβRII significantly increased TGF-β induction of Smad3 phosphorylation and stimulated expression of ECM components. Reduced expression of TβRII and ECM components in response to reduced fibroblast size/mechanical force was fully reversed by restoring size/mechanical force. Reduced fibroblast size was associated with reduced expression of TβRII and diminished ECM production, in aged human skin. Taken together, these data reveal a novel mechanism that provides a molecular basis for loss of dermal ECM, with concomitant increased fragility, which is a prominent feature of human skin aging.
Keywords: TGF-β type II receptor, TGF-β/Smad; aging; cell size; extracellular matrix; mechanotransduction.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

