Harnessing Redox Cross-Reactivity To Profile Distinct Cysteine Modifications
- PMID: 26780921
- PMCID: PMC4883004
- DOI: 10.1021/jacs.5b06806
Harnessing Redox Cross-Reactivity To Profile Distinct Cysteine Modifications
Abstract
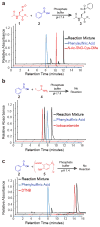
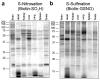
Cysteine S-nitrosation and S-sulfination are naturally occurring post-translational modifications (PTMs) on proteins induced by physiological signals and redox stress. Here we demonstrate that sulfinic acids and nitrosothiols react to form a stable thiosulfonate bond, and leverage this reactivity using sulfinate-linked probes to enrich and annotate hundreds of endogenous S-nitrosated proteins. In physiological buffers, sulfinic acids do not react with iodoacetamide or disulfides, enabling selective alkylation of free thiols and site-specific analysis of S-nitrosation. In parallel, S-nitrosothiol-linked probes enable enrichment and detection of endogenous S-sulfinated proteins, confirming that a single sulfinic acid can react with a nitrosothiol to form a thiosulfonate linkage. Using this approach, we find that hydrogen peroxide addition increases S-sulfination of human DJ-1 (PARK7) at Cys106, whereas Cys46 and Cys53 are fully oxidized to sulfonic acids. Comparative gel-based analysis of different mouse tissues reveals distinct profiles for both S-nitrosation and S-sulfination. Quantitative proteomic analysis demonstrates that both S-nitrosation and S-sulfination are widespread, yet exhibit enhanced occupancy on select proteins, including thioredoxin, peroxiredoxins, and other validated redox active proteins. Overall, we present a direct, bidirectional method to profile select redox cysteine modifications based on the unique nucleophilicity of sulfinic acids.
Figures
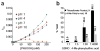





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

