Apoptosis, autophagy and unfolded protein response pathways in Arbovirus replication and pathogenesis
- PMID: 26781343
- PMCID: PMC4836210
- DOI: 10.1017/erm.2015.19
Apoptosis, autophagy and unfolded protein response pathways in Arbovirus replication and pathogenesis
Abstract
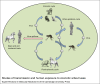
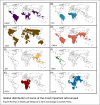
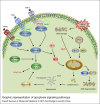
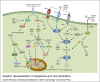
Arboviruses are pathogens that widely affect the health of people in different communities around the world. Recently, a few successful approaches toward production of effective vaccines against some of these pathogens have been developed, but treatment and prevention of the resulting diseases remain a major health and research concern. The arbovirus infection and replication processes are complex, and many factors are involved in their regulation. Apoptosis, autophagy and the unfolded protein response (UPR) are three mechanisms that are involved in pathogenesis of many viruses. In this review, we focus on the importance of these pathways in the arbovirus replication and infection processes. We provide a brief introduction on how apoptosis, autophagy and the UPR are initiated and regulated, and then discuss the involvement of these pathways in regulation of arbovirus pathogenesis.
Figures



References
-
- Gubler D.J. (2002) The global emergence/resurgence of arboviral diseases as public health problems. Archives of Medical Research 33, 330–342 - PubMed
-
- Calisher C. and Karabatsos N. (1988) Arbovirus serogroups: definition and geographic distribution. The Arboviruses: Epidemiology and Ecology 1, 19–57
-
- Heinz F.X. et al. (2000) Family Flaviviridae, Academic Press, San Diego, California
-
- Weaver S. et al. (2000) Family Togaviridae, Academic Press, San Diego, CA, USA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

