Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down, Edwards and Patau syndromes: a systematic review and meta-analysis
- PMID: 26781507
- PMCID: PMC4735304
- DOI: 10.1136/bmjopen-2015-010002
Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down, Edwards and Patau syndromes: a systematic review and meta-analysis
Abstract
Objective: To measure test accuracy of non-invasive prenatal testing (NIPT) for Down, Edwards and Patau syndromes using cell-free fetal DNA and identify factors affecting accuracy.
Design: Systematic review and meta-analysis of published studies.
Data sources: PubMed, Ovid Medline, Ovid Embase and the Cochrane Library published from 1997 to 9 February 2015, followed by weekly autoalerts until 1 April 2015.
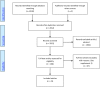
Eligibility criteria for selecting studies: English language journal articles describing case-control studies with ≥ 15 trisomy cases or cohort studies with ≥ 50 pregnant women who had been given NIPT and a reference standard.
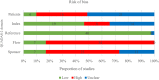
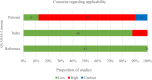
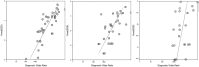
Results: 41, 37 and 30 studies of 2012 publications retrieved were included in the review for Down, Edwards and Patau syndromes. Quality appraisal identified high risk of bias in included studies, funnel plots showed evidence of publication bias. Pooled sensitivity was 99.3% (95% CI 98.9% to 99.6%) for Down, 97.4% (95.8% to 98.4%) for Edwards, and 97.4% (86.1% to 99.6%) for Patau syndrome. The pooled specificity was 99.9% (99.9% to 100%) for all three trisomies. In 100,000 pregnancies in the general obstetric population we would expect 417, 89 and 40 cases of Downs, Edwards and Patau syndromes to be detected by NIPT, with 94, 154 and 42 false positive results. Sensitivity was lower in twin than singleton pregnancies, reduced by 9% for Down, 28% for Edwards and 22% for Patau syndrome. Pooled sensitivity was also lower in the first trimester of pregnancy, in studies in the general obstetric population, and in cohort studies with consecutive enrolment.
Conclusions: NIPT using cell-free fetal DNA has very high sensitivity and specificity for Down syndrome, with slightly lower sensitivity for Edwards and Patau syndrome. However, it is not 100% accurate and should not be used as a final diagnosis for positive cases.
Trial registration number: CRD42014014947.
Keywords: REPRODUCTIVE MEDICINE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
