Endothelin-1 downregulates sperm phagocytosis by neutrophils in vitro: A physiological implication in bovine oviduct immunity
- PMID: 26781611
- PMCID: PMC4848572
- DOI: 10.1262/jrd.2015-112
Endothelin-1 downregulates sperm phagocytosis by neutrophils in vitro: A physiological implication in bovine oviduct immunity
Abstract
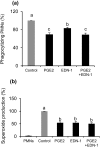
The oviduct is an active contractile tube that provides the proper environment for sperm transport, capacitation and survival. Oviductal contractions are regulated by autocrine/paracrine secretion of several factors, such as prostaglandins (PGs) and endothelin-1 (EDN-1). We have previously shown that during the preovulatory stage, sperm are exposed to polymorphonuclear neutrophils (PMNs) in the bovine oviduct, and the bovine oviduct epithelial cells (BOECs) secrete molecules including PGE2 that suppress sperm phagocytosis by PMNs in vitro. In this study, we investigated the possible effects of EDN-1 on the phagocytic activity of PMNs toward sperm. The local concentrations of EDN-1 in oviduct fluid and BOEC culture medium ranged from 10(-10) to 10(-11) M as determined by EIA. Phagocytosis and superoxide production were assayed by co-incubation of sperm pretreated to induce capacitation with PMNs exposed to EDN-1 (0, 10(-11), 10(-10), 10(-9), and 10(-8) M) for 2 h. EDN-1 suppressed dose dependently (10(-11) to 10(-8) M) the phagocytic activity for sperm and superoxide production of PMNs in response to capacitated sperm. Moreover, this suppression was eliminated by an ETB receptor antagonist (BQ-788). EDN-1 suppressed mRNA expression of EDN-1 and ETB but not ETA receptors in PMNs, suggesting the ETB receptor-mediated pathway. Scanning electron microscopic observation revealed that incubation of PMNs with EDN-1 (10(-9) M) completely suppressed the formation of DNA-based neutrophil extracellular traps for sperm entanglement. The results provide evidence indicating that EDN-1 may be involved in the protection of sperm from phagocytosis by PMNs in the bovine oviduct, supporting sperm survival until fertilization.
Figures

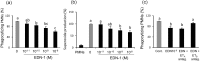
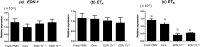

Similar articles
-
Bovine oviduct epithelial cells suppress the phagocytic activity of neutrophils towards sperm but not for bacteria in vitro: Immunofluorescence and electron microscopic observations.Histol Histopathol. 2020 Jun;35(6):589-597. doi: 10.14670/HH-18-172. Epub 2019 Oct 17. Histol Histopathol. 2020. PMID: 31621887
-
Bovine oviduct epithelial cells downregulate phagocytosis of sperm by neutrophils: prostaglandin E2 as a major physiological regulator.Reproduction. 2013 Dec 24;147(2):211-9. doi: 10.1530/REP-13-0375. Print 2014 Feb. Reproduction. 2013. PMID: 24255155
-
An acute-phase protein as a regulator of sperm survival in the bovine oviduct: alpha 1-acid-glycoprotein impairs neutrophil phagocytosis of sperm in vitro.J Reprod Dev. 2014;60(5):342-8. doi: 10.1262/jrd.2014-049. Epub 2014 Jun 13. J Reprod Dev. 2014. PMID: 24931131 Free PMC article.
-
Local immune system in oviduct physiology and pathophysiology: attack or tolerance?Domest Anim Endocrinol. 2016 Jul;56 Suppl:S204-11. doi: 10.1016/j.domaniend.2016.02.005. Domest Anim Endocrinol. 2016. PMID: 27345318 Review.
-
Involvement of oviduct in sperm capacitation and oocyte development in pigs.Reprod Suppl. 2001;58:129-45. Reprod Suppl. 2001. PMID: 11980185 Review.
Cited by
-
Analysis of the Combined Effects of a Novel Combination of Hypersmin, Pumpkin Seed and Amaranthus Extracts in an In Vitro Model of Chronic Venous Insufficiency.Nutrients. 2025 May 26;17(11):1807. doi: 10.3390/nu17111807. Nutrients. 2025. PMID: 40507076 Free PMC article.
-
A Higher Abundance of O-Linked Glycans Confers a Selective Advantage to High Fertile Buffalo Spermatozoa for Immune-Evasion From Neutrophils.Front Immunol. 2020 Aug 28;11:1928. doi: 10.3389/fimmu.2020.01928. eCollection 2020. Front Immunol. 2020. PMID: 32983120 Free PMC article.
-
Bovine oviduct epithelial cells suppress the phagocytic activity of neutrophils towards sperm but not for bacteria in vitro: Immunofluorescence and electron microscopic observations.Histol Histopathol. 2020 Jun;35(6):589-597. doi: 10.14670/HH-18-172. Epub 2019 Oct 17. Histol Histopathol. 2020. PMID: 31621887
-
An autoregressive logistic model to predict the reciprocal effects of oviductal fluid components on in vitro spermophagy by neutrophils in cattle.Sci Rep. 2017 Jun 30;7(1):4482. doi: 10.1038/s41598-017-04841-z. Sci Rep. 2017. PMID: 28667317 Free PMC article.
-
Effect of pH and neutrophil count on the motility and persistence of spermatozoa in the vagina of candidiasis rat models.Narra J. 2024 Aug;4(2):e823. doi: 10.52225/narra.v4i2.823. Epub 2024 Jun 21. Narra J. 2024. PMID: 39280289 Free PMC article.
References
-
- Ellington JE. The bovine oviduct and its role in reproduction: a review of the literature. Cornell Vet 1991; 81: 313–328. - PubMed
-
- Kölle S, Reese S, Kummer W. New aspects of gamete transport, fertilization, and embryonic development in the oviduct gained by means of live cell imaging. Theriogenology 2010; 73: 786–795. - PubMed
-
- Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol 2010; 88: 257–268. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

