Insulin-like growth factor 2 mRNA-binding protein-3 as a marker for distinguishing between cutaneous squamous cell carcinoma and keratoacanthoma
- PMID: 26782292
- PMCID: PMC4750532
- DOI: 10.3892/ijo.2016.3323
Insulin-like growth factor 2 mRNA-binding protein-3 as a marker for distinguishing between cutaneous squamous cell carcinoma and keratoacanthoma
Abstract
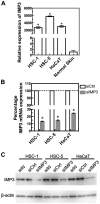
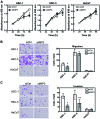
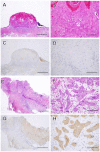
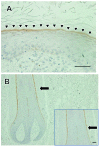
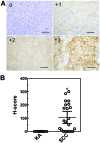
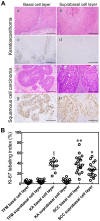
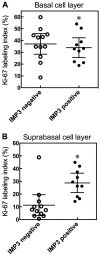
In the histopathological diagnosis of cutaneous tumors, the differential diagnosis of squamous cell carcinoma (SCC) with crateriform architecture and keratoacanthoma (KA) is often difficult so an accurate understanding of the biological features and the identification of reliable markers of SCC and KA are crucial issues. Insulin-like growth factor 2 mRNA-binding protein-3 (IGF2BP3, also known as IMP3) is thought of as a bona fide oncofetal protein, which is overexpressed and is involved in cell proliferation, migration, and invasion in several kinds of tumors. However, the role of IMP3 in cutaneous SCC and KA has not been well studied. Therefore, we focused on studying the biological functions of IMP3 in SCC and KA. In human skin SCC cell lines, HSC-1 and HSC-5, and the human keratinocyte cell line, HaCaT, IMP3 mRNA levels were significantly higher than that of normal human skin. The knockdown of IMP3 expression reduced the proliferation of HSC-1, and significantly reduced invasion by HSC-1 and HSC-5. In contrast, the knockdown of IMP3 did not significantly affect invasion by HaCaT cells. In immunohistochemical studies of SCC and KA tissues, the Ki-67 labeling index (LI) of the suprabasal cell layer was significantly higher in SCC, compared with KA tissues and the tumor-free margin (TFM) adjacent to SCC and KA. Most SCC tissues stained strongly positive for IMP3, but KA tissues and TFM were mostly negative for IMP3. The Ki-67 LI of the IMP3-positive group was significantly higher than that of the IMP3-negative group in the suprabasal cell layer of SCC. These results suggest that IMP3 plays an important role in proliferation and, more significantly, in the invasion of SCC, and may be a suitable marker for the histopathological diagnosis of SCC with a crateriform architecture and KA. Furthermore, IMP3 may potentially be a new therapeutic target for SCC.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

