Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double-strand breaks in human ovarian cancer cells
- PMID: 26782830
- PMCID: PMC4768956
- DOI: 10.3892/mmr.2016.4763
Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double-strand breaks in human ovarian cancer cells
Abstract
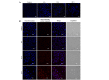
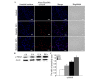
The mechanisms underlying myricetin-induced cancer cell apoptosis remain to be elucidated. Certain previous studies have shown that myricetin induces apoptosis through the mitochondrial pathway. Apoptosis, however, can also be induced by other classical pathways, including endoplasmic reticulum (ER) stress and DNA double‑strand breaks (DSBs). The aim of the present study was to assess whether these two apoptotic pathways are involved in myricetin‑induced cell death in SKOV3 ovarian cancer cells. The results revealed that treatment with myricetin inhibited viability of SKOV3 cells in a dose‑dependent manner. Myricetin induced nuclear chromatin condensation and fragmentation, and also upregulated the protein levels of active caspase 3 in a time‑dependent manner. In addition, myricetin upregulated ER stress‑associated proteins, glucose‑regulated protein‑78 and C/EBP homologous protein in SKOV3 cells. Phosphorylation of H2AX, a marker of DNA DSBs, was revealed to be upregulated in myricetin-treated cells. The data indicated that myricetin induces DNA DSBs and ER stress, which leads to apoptosis in SKOV3 cells.
Figures


References
-
- Tumanian SV, Iartseva DV. Effect of hepatic functional activity of the liver and endogenous intoxication in patients with ovarian cancer. Khirurgiia (Mosk) 2014:45–47. In Russian. - PubMed
-
- Shibata Y. Initial safety and efficacy of cisplatin and gemcitabine combination chemotherapy for unresectable biliary tract cancer. Gan To Kagaku Ryoho. 2014;41:2599–2602. - PubMed
-
- Yang J, Shi Y, He X, Dong M, Zhang C, Liu P, Zhou S, Qin Y, Gui L, Yang S, Sun Y. A pilot study of the safety and efficacy of dexamethasone, ifosfamide, methotrexate and gemcitabine chemotherapy for natural killer/T-cell lymphoma. Leuk Lymphoma. 2015;56:2218–2221. doi: 10.3109/10428194.2014.999323. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

