Ceramide Synthase 6 Is a Novel Target of Methotrexate Mediating Its Antiproliferative Effect in a p53-Dependent Manner
- PMID: 26783755
- PMCID: PMC4718595
- DOI: 10.1371/journal.pone.0146618
Ceramide Synthase 6 Is a Novel Target of Methotrexate Mediating Its Antiproliferative Effect in a p53-Dependent Manner
Abstract
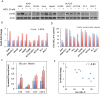
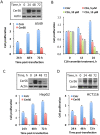
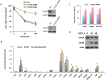
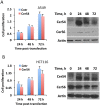
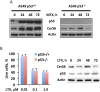
We previously reported that ceramide synthase 6 (CerS6) is elevated in response to folate stress in cancer cells, leading to enhanced production of C16-ceramide and apoptosis. Antifolate methotrexate (MTX), a drug commonly used in chemotherapy of several types of cancer, is a strong inhibitor of folate metabolism. Here we investigated whether this drug targets CerS6. We observed that CerS6 protein was markedly elevated in several cancer cell lines treated with MTX. In agreement with the enzyme elevation, its product C16-ceramide was also strongly elevated, so as several other ceramide species. The increase in C16-ceramide, however, was eliminated in MTX-treated cells lacking CerS6 through siRNA silencing, while the increase in other ceramides sustained. Furthermore, the siRNA silencing of CerS6 robustly protected A549 lung adenocarcinoma cells from MTX toxicity, while the silencing of another ceramide synthase, CerS4, which was also responsive to folate stress in our previous study, did not interfere with the MTX effect. The rescue effect of CerS6 silencing upon MTX treatment was further confirmed in HCT116 and HepG2 cell lines. Interestingly, CerS6 itself, but not CerS4, induced strong antiproliferative effect in several cancer cell lines if elevated by transient transfection. The effect of MTX on CerS6 elevation was likely p53 dependent, which is in agreement with the hypothesis that the protein is a transcriptional target of p53. In line with this notion, lometrexol, the antifolate inducing cytotoxicity through the p53-independent mechanism, did not affect CerS6 levels. We have also found that MTX induces the formation of ER aggregates, enriched with CerS6 protein. We further demonstrated that such aggregation requires CerS6 and suggests that it is an indication of ER stress. Overall, our study identified CerS6 and ceramide pathways as a novel MTX target.
Conflict of interest statement
Figures


Similar articles
-
CerS6 Is a Novel Transcriptional Target of p53 Protein Activated by Non-genotoxic Stress.J Biol Chem. 2016 Aug 5;291(32):16586-96. doi: 10.1074/jbc.M116.716902. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302066 Free PMC article.
-
Folate stress induces apoptosis via p53-dependent de novo ceramide synthesis and up-regulation of ceramide synthase 6.J Biol Chem. 2013 May 3;288(18):12880-90. doi: 10.1074/jbc.M113.461798. Epub 2013 Mar 21. J Biol Chem. 2013. PMID: 23519469 Free PMC article.
-
The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach.Mol Metab. 2019 Mar;21:36-50. doi: 10.1016/j.molmet.2018.12.008. Epub 2019 Jan 2. Mol Metab. 2019. PMID: 30655217 Free PMC article.
-
Diverse functions of ceramide in cancer cell death and proliferation.Adv Cancer Res. 2013;117:37-58. doi: 10.1016/B978-0-12-394274-6.00002-9. Adv Cancer Res. 2013. PMID: 23290776 Review.
-
The Role of Longevity Assurance Homolog 2/Ceramide Synthase 2 in Bladder Cancer.Int J Mol Sci. 2023 Oct 27;24(21):15668. doi: 10.3390/ijms242115668. Int J Mol Sci. 2023. PMID: 37958652 Free PMC article. Review.
Cited by
-
Co-targeting of specific epigenetic regulators in combination with CDC7 potently inhibit melanoma growth.iScience. 2022 Jul 15;25(8):104752. doi: 10.1016/j.isci.2022.104752. eCollection 2022 Aug 19. iScience. 2022. PMID: 35942091 Free PMC article.
-
Methotrexate remediates spinal cord injury in vivo and in vitro via suppression of endoplasmic reticulum stress-induced apoptosis.Exp Ther Med. 2018 May;15(5):4191-4198. doi: 10.3892/etm.2018.5973. Epub 2018 Mar 20. Exp Ther Med. 2018. PMID: 29731818 Free PMC article.
-
Ceramide in Coronary Artery Disease: Troublesome or Helpful Future Tools in the Assessment of Risk Prediction and Therapy Effectiveness?Metabolites. 2025 Mar 1;15(3):168. doi: 10.3390/metabo15030168. Metabolites. 2025. PMID: 40137133 Free PMC article. Review.
-
Restoration of ceramide de novo synthesis by the synthetic retinoid ST1926 as it induces adult T-cell leukemia cell death.Biosci Rep. 2020 Oct 30;40(10):BSR20200050. doi: 10.1042/BSR20200050. Biosci Rep. 2020. PMID: 33048123 Free PMC article.
-
CerS6 Is a Novel Transcriptional Target of p53 Protein Activated by Non-genotoxic Stress.J Biol Chem. 2016 Aug 5;291(32):16586-96. doi: 10.1074/jbc.M116.716902. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302066 Free PMC article.
References
-
- Goldman ID, Chattopadhyay S, Zhao R, Moran R. The antifolates: evolution, new agents in the clinic, and how targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs. Curr Opin Investig Drugs. 2010;11(12):1409–23. Epub 2010/12/15. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

