Correlative Gene Expression to Protective Seroconversion in Rift Valley Fever Vaccinates
- PMID: 26783758
- PMCID: PMC4718665
- DOI: 10.1371/journal.pone.0147027
Correlative Gene Expression to Protective Seroconversion in Rift Valley Fever Vaccinates
Erratum in
-
Correction: Correlative Gene Expression to Protective Seroconversion In Rift Valley Vaccinates.PLoS One. 2016 May 23;11(5):e0156469. doi: 10.1371/journal.pone.0156469. eCollection 2016. PLoS One. 2016. PMID: 27214231 Free PMC article.
Abstract
Rift Valley fever Virus (RVFV), a negative-stranded RNA virus, is the etiological agent of the vector-borne zoonotic disease, Rift Valley fever (RVF). In both humans and livestock, protective immunity can be achieved through vaccination. Earlier and more recent vaccine trials in cattle and sheep demonstrated a strong neutralizing antibody and total IgG response induced by the RVF vaccine, authentic recombinant MP-12 (arMP-12). From previous work, protective immunity in sheep and cattle vaccinates normally occurs from 7 to 21 days after inoculation with arMP-12. While the serology and protective response induced by arMP-12 has been studied, little attention has been paid to the underlying molecular and genetic events occurring prior to the serologic immune response. To address this, we isolated RNA from whole blood of vaccinated calves over a time course of 21 days before and after vaccination with arMP-12. The time course RNAs were sequenced by RNASeq and bioinformatically analyzed. Our results revealed time-dependent activation or repression of numerous gene ontologies and pathways related to the vaccine induced immune response and its regulation. Additional bioinformatic analyses identified a correlative relationship between specific host immune response genes and protective immunity prior to the detection of protective serum neutralizing antibody responses. These results contribute an important proof of concept for identifying molecular and genetic components underlying the immune response to RVF vaccination and protection prior to serologic detection.
Conflict of interest statement
Figures
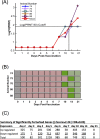








References
-
- Daubney R H, JR. Enzootic hepatitis or Rift Valley fever: an underscribed virus disease of sheep cattle and man from east Africa. Journal of Pathogenic Bacteriology. 1931;34:545–79.
-
- Ahmed J, Bouloy M, Ergonul O, Fooks A, Paweska J, Chevalier V, et al. International network for capacity building for the control of emerging viral vector-borne zoonotic diseases: ARBO-ZOONET. Euro surveillance: bulletin europeen sur les maladies transmissibles = European communicable disease bulletin. 2009;14(12). Epub 2009/04/04. . - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

