Iron in Multiple Sclerosis and Its Noninvasive Imaging with Quantitative Susceptibility Mapping
- PMID: 26784172
- PMCID: PMC4730342
- DOI: 10.3390/ijms17010100
Iron in Multiple Sclerosis and Its Noninvasive Imaging with Quantitative Susceptibility Mapping
Abstract
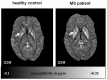
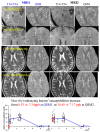
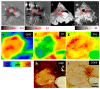
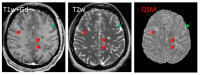
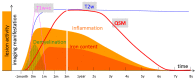
Iron is considered to play a key role in the development and progression of Multiple Sclerosis (MS). In particular, iron that accumulates in myeloid cells after the blood-brain barrier (BBB) seals may contribute to chronic inflammation, oxidative stress and eventually neurodegeneration. Magnetic resonance imaging (MRI) is a well-established tool for the non-invasive study of MS. In recent years, an advanced MRI method, quantitative susceptibility mapping (QSM), has made it possible to study brain iron through in vivo imaging. Moreover, immunohistochemical investigations have helped defining the lesional and cellular distribution of iron in MS brain tissue. Imaging studies in MS patients and of brain tissue combined with histological studies have provided important insights into the role of iron in inflammation and neurodegeneration in MS.
Keywords: MRI; MS lesion; deep grey matter (DGM); quantitative susceptibility mapping (QSM).
Figures


Similar articles
-
Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: Interpreting positive susceptibility and the presence of iron.Magn Reson Med. 2015 Aug;74(2):564-70. doi: 10.1002/mrm.25420. Epub 2014 Aug 18. Magn Reson Med. 2015. PMID: 25137340 Free PMC article.
-
Iron Mapping in Multiple Sclerosis.Neuroimaging Clin N Am. 2017 May;27(2):335-342. doi: 10.1016/j.nic.2016.12.003. Epub 2017 Jan 20. Neuroimaging Clin N Am. 2017. PMID: 28391790 Review.
-
Validation of quantitative susceptibility mapping with Perls' iron staining for subcortical gray matter.Neuroimage. 2015 Jan 15;105:486-92. doi: 10.1016/j.neuroimage.2014.11.010. Epub 2014 Nov 8. Neuroimage. 2015. PMID: 25462797
-
Changes of deep gray matter magnetic susceptibility over 2 years in multiple sclerosis and healthy control brain.Neuroimage Clin. 2017 Apr 13;18:1007-1016. doi: 10.1016/j.nicl.2017.04.008. eCollection 2018. Neuroimage Clin. 2017. PMID: 29868452 Free PMC article.
-
MRI assessment of iron deposition in multiple sclerosis.J Magn Reson Imaging. 2011 Jul;34(1):13-21. doi: 10.1002/jmri.22590. J Magn Reson Imaging. 2011. PMID: 21698703 Review.
Cited by
-
A Comparison of Magnetic Resonance Imaging Methods to Assess Multiple Sclerosis Lesions: Implications for Patient Characterization and Clinical Trial Design.Diagnostics (Basel). 2021 Dec 30;12(1):77. doi: 10.3390/diagnostics12010077. Diagnostics (Basel). 2021. PMID: 35054244 Free PMC article.
-
Blood Perfusion and Cellular Microstructural Changes Associated With Iron Deposition in Multiple Sclerosis Lesions.Front Neurol. 2019 Jul 11;10:747. doi: 10.3389/fneur.2019.00747. eCollection 2019. Front Neurol. 2019. PMID: 31354613 Free PMC article.
-
Identification of Key Ferroptosis-Related Genes in the Peripheral Blood of Patients with Relapsing-Remitting Multiple Sclerosis and Its Diagnostic Value.Int J Mol Sci. 2023 Mar 29;24(7):6399. doi: 10.3390/ijms24076399. Int J Mol Sci. 2023. PMID: 37047371 Free PMC article.
-
Mapping of thalamic magnetic susceptibility in multiple sclerosis indicates decreasing iron with disease duration: A proposed mechanistic relationship between inflammation and oligodendrocyte vitality.Neuroimage. 2018 Feb 15;167:438-452. doi: 10.1016/j.neuroimage.2017.10.063. Epub 2017 Oct 31. Neuroimage. 2018. PMID: 29097315 Free PMC article.
-
Automated Quantitative Susceptibility and Morphometry MR Study: Feasibility and Interrelation Between Clinical Score, Lesion Load, Deep Grey Matter and Normal-Appearing White Matter in Multiple Sclerosis.Diagnostics (Basel). 2024 Nov 27;14(23):2669. doi: 10.3390/diagnostics14232669. Diagnostics (Basel). 2024. PMID: 39682577 Free PMC article.
References
-
- Dexter D.T., Carayon A., Javoy-Agid F., Agid Y., Wells F.R., Daniel S.E., Lees A.J., Jenner P., Marsden C.D. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

