Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin
- PMID: 26784174
- PMCID: PMC4730345
- DOI: 10.3390/ijms17010103
Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin
Abstract
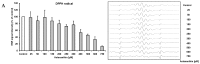
Astaxanthin is a coloring agent which is used as a feed additive in aquaculture nutrition. Recently, potential health benefits of astaxanthin have been discussed which may be partly related to its free radical scavenging and antioxidant properties. Our electron spin resonance (ESR) and spin trapping data suggest that synthetic astaxanthin is a potent free radical scavenger in terms of diphenylpicryl-hydrazyl (DPPH) and galvinoxyl free radicals. Furthermore, astaxanthin dose-dependently quenched singlet oxygen as determined by photon counting. In addition to free radical scavenging and singlet oxygen quenching properties, astaxanthin induced the antioxidant enzyme paroxoanase-1, enhanced glutathione concentrations and prevented lipid peroxidation in cultured hepatocytes. Present results suggest that, beyond its coloring properties, synthetic astaxanthin exhibits free radical scavenging, singlet oxygen quenching, and antioxidant activities which could probably positively affect animal and human health.
Keywords: antioxidant; astaxanthin; electron spin resonance spectroscopy; free radical scavenging.
Figures





Similar articles
-
Free radical scavenging and antioxidant activity of betanin: electron spin resonance spectroscopy studies and studies in cultured cells.Food Chem Toxicol. 2014 Nov;73:119-26. doi: 10.1016/j.fct.2014.08.007. Epub 2014 Aug 23. Food Chem Toxicol. 2014. PMID: 25152328
-
Direct superoxide anion scavenging by a disodium disuccinate astaxanthin derivative: Relative efficacy of individual stereoisomers versus the statistical mixture of stereoisomers by electron paramagnetic resonance imaging.Biochem Biophys Res Commun. 2003 Aug 1;307(3):704-12. doi: 10.1016/s0006-291x(03)01248-8. Biochem Biophys Res Commun. 2003. PMID: 12893281
-
Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites.J Agric Food Chem. 2007 Oct 17;55(21):8516-22. doi: 10.1021/jf071848a. Epub 2007 Sep 26. J Agric Food Chem. 2007. PMID: 17894451
-
On the Neuroprotective Role of Astaxanthin: New Perspectives?Mar Drugs. 2018 Jul 24;16(8):247. doi: 10.3390/md16080247. Mar Drugs. 2018. PMID: 30042358 Free PMC article. Review.
-
Potential Anti-Atherosclerotic Properties of Astaxanthin.Mar Drugs. 2016 Feb 5;14(2):35. doi: 10.3390/md14020035. Mar Drugs. 2016. PMID: 26861359 Free PMC article. Review.
Cited by
-
Carbon Source Influences Antioxidant, Antiglycemic, and Antilipidemic Activities of Haloferax mediterranei Carotenoid Extracts.Mar Drugs. 2022 Oct 24;20(11):659. doi: 10.3390/md20110659. Mar Drugs. 2022. PMID: 36354982 Free PMC article.
-
Methionine Augments Antioxidant Activity of Rice Protein during Gastrointestinal Digestion.Int J Mol Sci. 2019 Feb 17;20(4):868. doi: 10.3390/ijms20040868. Int J Mol Sci. 2019. PMID: 30781587 Free PMC article.
-
High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice.Nutrients. 2019 May 31;11(6):1244. doi: 10.3390/nu11061244. Nutrients. 2019. PMID: 31159211 Free PMC article.
-
Effect of a combination of astaxanthin supplementation, heat stress, and intermittent reloading on satellite cells during disuse muscle atrophy.J Zhejiang Univ Sci B. 2018 Nov.;19(11):844-852. doi: 10.1631/jzus.B1800076. J Zhejiang Univ Sci B. 2018. PMID: 30387334 Free PMC article.
-
Enhanced Carotenoid Production in Chlamydomonas reinhardtii by Overexpression of Endogenousand Exogenous Beta-Carotene Ketolase (BKT) Genes.Int J Mol Sci. 2023 Jul 13;24(14):11382. doi: 10.3390/ijms241411382. Int J Mol Sci. 2023. PMID: 37511141 Free PMC article.
References
-
- Jiao G., Hui J.P.M., Burton I.W., Thibault M.-H., Pelletier C., Boudreau J., Tchoukanova N., Subramanian B., Djaoued Y., Ewart S., et al. Characterization of shrimp oil from Pandalus borealis by high performance liquid chromatography and high resolution mass spectrometry. Mar. Drugs. 2015;13:3849–3876. doi: 10.3390/md13063849. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

