DYRK1B as therapeutic target in Hedgehog/GLI-dependent cancer cells with Smoothened inhibitor resistance
- PMID: 26784250
- PMCID: PMC4872774
- DOI: 10.18632/oncotarget.6910
DYRK1B as therapeutic target in Hedgehog/GLI-dependent cancer cells with Smoothened inhibitor resistance
Abstract
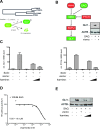
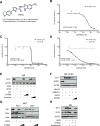
A wide range of human malignancies displays aberrant activation of Hedgehog (HH)/GLI signaling, including cancers of the skin, brain, gastrointestinal tract and hematopoietic system. Targeting oncogenic HH/GLI signaling with small molecule inhibitors of the essential pathway effector Smoothened (SMO) has shown remarkable therapeutic effects in patients with advanced and metastatic basal cell carcinoma. However, acquired and de novo resistance to SMO inhibitors poses severe limitations to the use of SMO antagonists and urgently calls for the identification of novel targets and compounds.Here we report on the identification of the Dual-Specificity-Tyrosine-Phosphorylation-Regulated Kinase 1B (DYRK1B) as critical positive regulator of HH/GLI signaling downstream of SMO. Genetic and chemical inhibition of DYRK1B in human and mouse cancer cells resulted in marked repression of HH signaling and GLI1 expression, respectively. Importantly, DYRK1B inhibition profoundly impaired GLI1 expression in both SMO-inhibitor sensitive and resistant settings. We further introduce a novel small molecule DYRK1B inhibitor, DYRKi, with suitable pharmacologic properties to impair SMO-dependent and SMO-independent oncogenic GLI activity. The results support the use of DYRK1B antagonists for the treatment of HH/GLI-associated cancers where SMO inhibitors fail to demonstrate therapeutic efficacy.
Keywords: DYRK1B; GLI transcription factors; Hedgehog/GLI signaling; Smoothened drug resistance; basal cell carcinoma.
Conflict of interest statement
MZ, SM, SH, JL, DV are or were employees of 4SC group (4SC Discovery GmbH and 4SC AG), 4SC Discovery GmbH is developing novel DYRK1B inhibitors for cancer therapy. 4SC AG and 4SC Discovery GmbH contributed to the costs for testing DYRK inhibitors. No other authors declared any conflict of interest.
Figures



References
-
- Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochimica et biophysica acta. 2010;1805:181–208. - PubMed
-
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of Hedgehog Pathway Inhibitors in Basal Cell Carcinoma. Molecular cancer therapeutics. 2015 - PubMed
-
- Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

