Pharmacological characterization of the first in class clinical candidate PF-05190457: a selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo
- PMID: 26784385
- PMCID: PMC4831304
- DOI: 10.1111/bph.13439
Pharmacological characterization of the first in class clinical candidate PF-05190457: a selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo
Abstract
Background and purpose: Ghrelin increases growth hormone secretion, gastric acid secretion, gastric motility and hunger but decreases glucose-dependent insulin secretion and insulin sensitivity in humans. Antagonizing the ghrelin receptor has potential as a therapeutic approach in the treatment of obesity and type 2 diabetes. Therefore, the aim was to pharmacologically characterize the novel small-molecule antagonist PF-05190457 and assess translational pharmacology ex vivo.
Experimental approach: Radioligand binding in filter and scintillation proximity assay formats were used to evaluate affinity, and europium-labelled GTP to assess functional activity. Rat vagal afferent firing and calcium imaging in dispersed islets were used as native tissues underlying food intake and insulin secretion respectively.
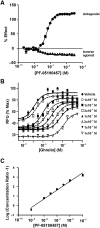
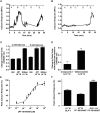
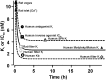
Key results: PF-05190457 was a potent and selective inverse agonist on constitutively active ghrelin receptors and acted as a competitive antagonist of ghrelin action, with a human Kd of 3 nM requiring 4 h to achieve equilibrium. Potency of PF-05190457 was similar across different species. PF-05190457 increased intracellular calcium within dispersed islets and increased vagal afferent firing in a concentration-dependent manner with similar potency but was threefold less potent as compared with the in vitro Ki in recombinant overexpressing cells. The effect of PF-05190457 on rodent islets was comparable with glibenclamide, but glucose-dependent and additive with the insulin secretagogue glucagon-like peptide-1.
Conclusions and implications: Together, these data provide the pharmacological in vitro and ex vivo characterization of the first ghrelin receptor inverse agonist, which has advanced into clinical trials to evaluate the therapeutic potential of blocking ghrelin receptors in obesity and type 2 diabetes.
© 2016 The British Pharmacological Society.
Figures


Similar articles
-
Pharmacokinetics and pharmacodynamics of PF-05190457: The first oral ghrelin receptor inverse agonist to be profiled in healthy subjects.Br J Clin Pharmacol. 2017 Feb;83(2):326-338. doi: 10.1111/bcp.13127. Epub 2016 Oct 29. Br J Clin Pharmacol. 2017. PMID: 27621150 Free PMC article. Clinical Trial.
-
Endocrine effects of the novel ghrelin receptor inverse agonist PF-5190457: Results from a placebo-controlled human laboratory alcohol co-administration study in heavy drinkers.Neuropharmacology. 2020 Jun 15;170:107788. doi: 10.1016/j.neuropharm.2019.107788. Epub 2019 Sep 23. Neuropharmacology. 2020. PMID: 31557492 Free PMC article. Clinical Trial.
-
The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study.Mol Psychiatry. 2020 Feb;25(2):461-475. doi: 10.1038/s41380-018-0064-y. Epub 2018 May 4. Mol Psychiatry. 2020. PMID: 29728704 Free PMC article. Clinical Trial.
-
Ghrelin regulation of glucose metabolism.J Neuroendocrinol. 2019 Jul;31(7):e12705. doi: 10.1111/jne.12705. Epub 2019 Apr 3. J Neuroendocrinol. 2019. PMID: 30849212 Free PMC article. Review.
-
Islet β-cell ghrelin signaling for inhibition of insulin secretion.Methods Enzymol. 2012;514:317-31. doi: 10.1016/B978-0-12-381272-8.00020-9. Methods Enzymol. 2012. PMID: 22975062 Review.
Cited by
-
Initial Pharmacological Characterization of a Major Hydroxy Metabolite of PF-5190457: Inverse Agonist Activity of PF-6870961 at the Ghrelin Receptor.J Pharmacol Exp Ther. 2023 Aug;386(2):117-128. doi: 10.1124/jpet.122.001393. Epub 2023 Jan 11. J Pharmacol Exp Ther. 2023. PMID: 36631279 Free PMC article.
-
An Emerging Role for Gut-Brain Signaling Involving Ghrelin in Chronic Stress.Adv Exp Med Biol. 2025;1477:205-227. doi: 10.1007/978-3-031-89525-8_7. Adv Exp Med Biol. 2025. PMID: 40442387 Review.
-
Development of Fluorinated Non-Peptidic Ghrelin Receptor Ligands for Potential Use in Molecular Imaging.Int J Mol Sci. 2017 Apr 5;18(4):768. doi: 10.3390/ijms18040768. Int J Mol Sci. 2017. PMID: 28379199 Free PMC article.
-
Ghrelin's Relationship to Blood Glucose.Endocrinology. 2019 May 1;160(5):1247-1261. doi: 10.1210/en.2019-00074. Endocrinology. 2019. PMID: 30874792 Free PMC article. Review.
-
"Sibling" battle or harmony: crosstalk between nesfatin-1 and ghrelin.Cell Mol Life Sci. 2022 Mar 3;79(3):169. doi: 10.1007/s00018-022-04193-6. Cell Mol Life Sci. 2022. PMID: 35239020 Free PMC article. Review.
References
-
- Arosia M, Ronchi CL, Gebbia C, Cappiello V, Beck‐Peccoz P, Peracchi M (2003). Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. J Clin Endocrinol Metab 88: 701–704. - PubMed
-
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. (2001). Ghrelin is an appetite‐stimulatory signal from stomach with structural resemblance to motilin. Gastroenterol 120: 337–345. - PubMed
-
- Banks WA, Tschöp M, Robinson SM, Heiman ML (2002). Extent and direction of ghrelin transport across the blood–brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302: 822–827. - PubMed
-
- Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, et al. (2007). Relationships between desacylated and acylated ghrelin and insulin sensitivity in metabolic syndrome. J Clin Endocrinol Metab 92: 3935–3940. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

