Lung VITAL: Rationale, design, and baseline characteristics of an ancillary study evaluating the effects of vitamin D and/or marine omega-3 fatty acid supplements on acute exacerbations of chronic respiratory disease, asthma control, pneumonia and lung function in adults
- PMID: 26784651
- PMCID: PMC4818187
- DOI: 10.1016/j.cct.2016.01.003
Lung VITAL: Rationale, design, and baseline characteristics of an ancillary study evaluating the effects of vitamin D and/or marine omega-3 fatty acid supplements on acute exacerbations of chronic respiratory disease, asthma control, pneumonia and lung function in adults
Abstract
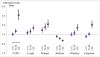
Laboratory and observational research studies suggest that vitamin D and marine omega-3 fatty acids may reduce risk for pneumonia, acute exacerbations of respiratory diseases including chronic obstructive lung disease (COPD) or asthma, and decline of lung function, but prevention trials with adequate dosing, adequate power, and adequate time to follow-up are lacking. The ongoing Lung VITAL study is taking advantage of a large clinical trial-the VITamin D and OmegA-3 TriaL (VITAL)--to conduct the first major evaluation of the influences of vitamin D and marine omega-3 fatty acid supplementation on pneumonia risk, respiratory exacerbation episodes, asthma control and lung function in adults. VITAL is a 5-year U.S.-wide randomized, double-blind, placebo-controlled, 2 × 2 factorial trial of supplementation with vitamin D3 ([cholecalciferol], 2000 IU/day) and marine omega-3 FA (Omacor® fish oil, eicosapentaenoic acid [EPA]+docosahexaenoic acid [DHA], 1g/day) for primary prevention of CVD and cancer among men and women, at baseline aged ≥50 and ≥55, respectively, with 5107 African Americans. In a subset of 1973 participants from 11 urban U.S. centers, lung function is measured before and two years after randomization. Yearly follow-up questionnaires assess incident pneumonia in the entire randomized population, and exacerbations of respiratory disease, asthma control and dyspnea in a subpopulation of 4314 randomized participants enriched, as shown in presentation of baseline characteristics, for respiratory disease, respiratory symptoms, and history of cigarette smoking. Self-reported pneumonia hospitalization will be confirmed by medical record review, and exacerbations will be confirmed by Center for Medicare and Medicaid Services data review.
Trial registration: ClinicalTrials.gov NCT01728571.
Keywords: COPD; Lung function; Omega-3 fatty acids; Randomized clinical trial; Respiratory symptoms; Vitamin D.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29(6):1224–1238. - PubMed
-
- Peto R, Speizer FE, Cochrane AL, Moore F, Fletcher CM, Tinker CM, Higgins IT, Gray RG, Richards SM, Gilliland J, Norman-Smith B. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation. Am Rev Respir Dis. 1983;128(3):491–500. - PubMed
-
- Hiemstra PS. Defensins and cathelicidins in inflammatory lung disease: beyond antimicrobial activity. Biochem Soc Trans. 2006;34(Pt 2):276–278. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

