Bcl-xL promotes metastasis independent of its anti-apoptotic activity
- PMID: 26785948
- PMCID: PMC4735924
- DOI: 10.1038/ncomms10384
Bcl-xL promotes metastasis independent of its anti-apoptotic activity
Abstract
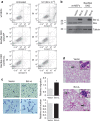
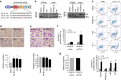
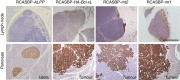
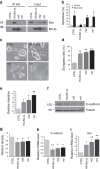
Bcl-xL suppresses mitochondria-mediated apoptosis and is frequently overexpressed in cancer to promote cancer cell survival. Bcl-xL also promotes metastasis. However, it is unclear whether this metastatic function is dependent on its anti-apoptotic activity in the mitochondria. Here we demonstrate that Bcl-xL promotes metastasis independent of its anti-apoptotic activity. We show that apoptosis-defective Bcl-xL mutants and an engineered Bcl-xL targeted to the nucleus promote epithelial-mesenchymal transition, migration, invasion and stemness in pancreatic neuroendocrine tumour (panNET) and breast cancer cell lines. However, Bcl-xL proteins targeted to the mitochondria or outside of the nucleus do not have these functions. We confirm our findings in spontaneous and xenograft mouse models. Furthermore, Bcl-xL exerts metastatic function through epigenetic modification of the TGFβ promoter to increase TGFβ signalling. Consistent with these findings, we detect nuclear Bcl-xL in human metastatic panNETs. Taken together, the metastatic function of Bcl-xL is independent of its anti-apoptotic activity and its residence in the mitochondria.
Figures
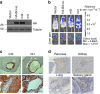
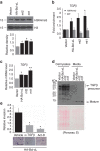
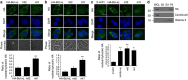
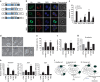
References
-
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). - PubMed
-
- Reed J. C. Dysregulation of apoptosis in cancer. J. Clin. Oncol. 17, 2941–2953 (1999). - PubMed
-
- Yin X. M., Oltvai Z. N. & Korsmeyer S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369, 321–323 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

