Structural Determinants of Oligomerization of the Aquaporin-4 Channel
- PMID: 26786101
- PMCID: PMC4807272
- DOI: 10.1074/jbc.M115.694729
Structural Determinants of Oligomerization of the Aquaporin-4 Channel
Abstract
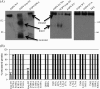
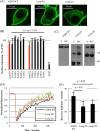
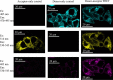
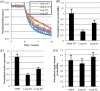
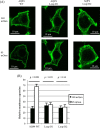
The aquaporin (AQP) family of integral membrane protein channels mediate cellular water and solute flow. Although qualitative and quantitative differences in channel permeability, selectivity, subcellular localization, and trafficking responses have been observed for different members of the AQP family, the signature homotetrameric quaternary structure is conserved. Using a variety of biophysical techniques, we show that mutations to an intracellular loop (loop D) of human AQP4 reduce oligomerization. Non-tetrameric AQP4 mutants are unable to relocalize to the plasma membrane in response to changes in extracellular tonicity, despite equivalent constitutive surface expression levels and water permeability to wild-type AQP4. A network of AQP4 loop D hydrogen bonding interactions, identified using molecular dynamics simulations and based on a comparative mutagenic analysis of AQPs 1, 3, and 4, suggest that loop D interactions may provide a general structural framework for tetrameric assembly within the AQP family.
Keywords: aquaporin; cellular regulation; oligomerization; protein translocation; water channel.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Jung J. S., Preston G. M., Smith B. L., Guggino W. B., and Agre P. (1994) Molecular structure of the water channel through aquaporin CHIP: the hourglass model. J. Biol. Chem. 269, 14648–14654 - PubMed
-
- Hub J. S., Grubmuller H., and de Groot B. L. (2009) Dynamics and energetics of permeation through aquaporins: what do we learn from molecular dynamics simulations? Handb. Exp. Pharmacol. 2009, 57–76 - PubMed
-
- Kaldenhoff R., Kai L., and Uehlein N. (2014) Aquaporins and membrane diffusion of CO2 in living organisms. Biochim. Biophys. Acta 1840, 1592–1595 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

