A Signaling Network Controlling Androgenic Repression of c-Fos Protein in Prostate Adenocarcinoma Cells
- PMID: 26786102
- PMCID: PMC4786693
- DOI: 10.1074/jbc.M115.694877
A Signaling Network Controlling Androgenic Repression of c-Fos Protein in Prostate Adenocarcinoma Cells
Abstract
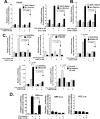
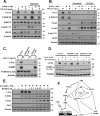
The transcription factor c-Fos controls many important cellular processes, including cell growth and apoptosis. c-Fos expression is rapidly elevated in the prostate upon castration-mediated androgen withdrawal through an undefined mechanism. Here we show that androgens (5α-dihydrotestosterone and R1881) suppress c-Fos protein and mRNA expression induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) or EGF in human prostate cancer (PCa) cell lines. Such suppression transpires through a transcriptional mechanism, predominantly at the proximal serum response element of the c-fos promoter. We show that androgen signaling suppresses TPA-induced c-Fos expression through repressing a PKC/MEK/ERK/ELK-1 signaling pathway. Moreover, our results support the hypothesis that p38(MAPK), PI3K, and PKCδ are involved in the androgenic regulation of c-Fos through controlling MEK/ERK. Stable silencing of c-Fos and PKCδ with shRNAs suggests that R1881 promotes cell death induced by low-dose TPA through a mechanism that is dependent on both PKCδ and loss of c-Fos expression. Reciprocally, loss of either PKCδ or c-Fos activates p38(MAPK) while suppressing the activation of ERK1/2. We also provide the first demonstration that R1881 permits cell death induced by low-dose TPA in the LNCaP androgen-dependent PCa cell line and that TPA-induced cell death is independent of exogenous androgen in the castration-resistant variants of LNCaP, C4-2 and C4-2B. Acquisition of androgen-independent killing by TPA correlates with activation of p38(MAPK), suppression of ERK1/2, and loss of c-Fos. These results provide new insights into androgenic control of c-Fos and use of PKC inhibitors in PCa therapy.
Keywords: Akt PKB; MAPK; PKC; androgen; androgen receptor; c-Fos; c-Jun transcription factor; p38 MAPK; phorbol ester; prostate cancer.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Tumor-promoting phorbol ester-induced cell death and gene expression in a human prostate adenocarcinoma cell line.Oncol Res. 1994;6(4-5):203-10. Oncol Res. 1994. PMID: 7841543
-
Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway.J Biol Chem. 2003 Sep 5;278(36):33753-62. doi: 10.1074/jbc.M303313200. Epub 2003 Jun 24. J Biol Chem. 2003. PMID: 12824193
-
Rapid signalling by androgen receptor in prostate cancer cells.Oncogene. 1999 Nov 4;18(46):6322-9. doi: 10.1038/sj.onc.1203032. Oncogene. 1999. PMID: 10597231
-
Molecular Mechanism of Tanshinone against Prostate Cancer.Molecules. 2022 Aug 30;27(17):5594. doi: 10.3390/molecules27175594. Molecules. 2022. PMID: 36080361 Free PMC article. Review.
-
Molecular oncogenesis of prostate adenocarcinoma: role of the human epidermal growth factor receptor 2 (HER-2/neu).Tumori. 2010 Sep-Oct;96(5):645-9. doi: 10.1177/030089161009600501. Tumori. 2010. PMID: 21302606 Review.
Cited by
-
Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3.Int J Mol Sci. 2016 May 14;17(5):738. doi: 10.3390/ijms17050738. Int J Mol Sci. 2016. PMID: 27187384 Free PMC article.
-
Hypoxia represses early responses of prostate and renal cancer cells to YM155 independent of HIF-1α and HIF-2α.Curr Res Pharmacol Drug Discov. 2021 Dec 23;3:100076. doi: 10.1016/j.crphar.2021.100076. eCollection 2022. Curr Res Pharmacol Drug Discov. 2021. PMID: 35005610 Free PMC article.
-
Characterization of HMGB1/2 Interactome in Prostate Cancer by Yeast Two Hybrid Approach: Potential Pathobiological Implications.Cancers (Basel). 2019 Nov 5;11(11):1729. doi: 10.3390/cancers11111729. Cancers (Basel). 2019. PMID: 31694235 Free PMC article.
-
Differential gene expression and hallmarks of stemness in epithelial cells of the developing rat epididymis.Cell Tissue Res. 2022 Aug;389(2):327-349. doi: 10.1007/s00441-022-03634-9. Epub 2022 May 20. Cell Tissue Res. 2022. PMID: 35590013
-
The histone demethylase KDM3A regulates the transcriptional program of the androgen receptor in prostate cancer cells.Oncotarget. 2017 May 2;8(18):30328-30343. doi: 10.18632/oncotarget.15681. Oncotarget. 2017. PMID: 28416760 Free PMC article.
References
-
- Cooke P. S., Young P., and Cunha G. R. (1991) Androgen receptor expression in developing male reproductive organs. Endocrinology 128, 2867–2873 - PubMed
-
- Heinlein C. A., and Chang C. (2004) Androgen receptor in prostate cancer. Endocr. Rev. 25, 276–308 - PubMed
-
- Huggins C., and Hodges C. V. (1972) Studies on prostatic cancer: I: the effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 22, 232–240 - PubMed
-
- Veldscholte J., Berrevoets C. A., Zegers N. D., van der Kwast T. H., Grootegoed J. A., and Mulder E. (1992) Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry 31, 7422–7430 - PubMed
-
- Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., and Sawyers C. L. (2004) Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

