AChE Inhibition-based Multi-target-directed Ligands, a Novel Pharmacological Approach for the Symptomatic and Disease-modifying Therapy of Alzheimer's Disease
- PMID: 26786145
- PMCID: PMC4876592
- DOI: 10.2174/1570159x14666160119094820
AChE Inhibition-based Multi-target-directed Ligands, a Novel Pharmacological Approach for the Symptomatic and Disease-modifying Therapy of Alzheimer's Disease
Abstract
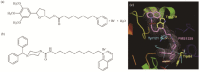
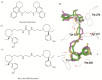
Alzheimer's disease (AD) is the most common form of dementia in elder people, characterised by a progressive decline in memory as a result of an impairment of cholinergic neurotransmission. To date acetylcholinesterase inhibitors (AChEIs) have become the most prescribed drugs for the symptomatic treatment of mild to moderate AD. However, the traditional "one molecule-one target" paradigm is not sufficient and appropriate to yield the desired therapeutic efficacy since multiple factors, such as amyloid-β (Aβ) deposits, neuroinflammation, oxidative stress, and decreased levels of acetylcholine (ACh) have been thought to play significant roles in the AD pathogenesis. New generation of multi-target drugs is earnestly demanded not only for ameliorating symptoms but also for modifying the disease. Herein, we delineated the catalytic and non-catalytic functions of AChE, and summarized the works of our group and others in research and development of novel AChEI-based multi-target-directed ligands (MTDLs), such as dual binding site AChEIs and multitarget AChEIs inhibiting Aβ aggregation, regulating Aβ procession, antagonizing platelet-activating factor (PAF) receptor, scavenging oxygen radical, chelating metal ions, inhibiting monoamine oxidase B (MAO-B), blocking N-methyl-D-aspartic acid (NMDA) receptor and others.
Figures

Similar articles
-
[Design of acetylcholinesterase inhibitor for Alzheimer's disease therapy: from multi-binding site inhibitors to multi-target directed ligands].Yao Xue Xue Bao. 2012 Mar;47(3):313-21. Yao Xue Xue Bao. 2012. PMID: 22645754 Review. Chinese.
-
Management of oxidative stress and other pathologies in Alzheimer's disease.Arch Toxicol. 2019 Sep;93(9):2491-2513. doi: 10.1007/s00204-019-02538-y. Epub 2019 Aug 22. Arch Toxicol. 2019. PMID: 31440798 Review.
-
Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer's disease.Curr Med Chem. 2008;15(24):2433-55. doi: 10.2174/092986708785909067. Curr Med Chem. 2008. PMID: 18855672 Review.
-
Novel small molecule therapeutic agents for Alzheimer disease: Focusing on BACE1 and multi-target directed ligands.Bioorg Chem. 2020 Apr;97:103649. doi: 10.1016/j.bioorg.2020.103649. Epub 2020 Feb 4. Bioorg Chem. 2020. PMID: 32101780 Review.
-
Dual-target inhibitors based on acetylcholinesterase: Novel agents for Alzheimer's disease.Eur J Med Chem. 2024 Dec 5;279:116810. doi: 10.1016/j.ejmech.2024.116810. Epub 2024 Sep 4. Eur J Med Chem. 2024. PMID: 39243456 Review.
Cited by
-
Assessment of in-vitro cholinesterase inhibitory and thrombolytic potential of bark and seed extracts of Tamarindus indica (L.) relevant to the treatment of Alzheimer's disease and clotting disorders.J Intercult Ethnopharmacol. 2017 Jan 3;6(1):115-120. doi: 10.5455/jice.20161229055750. eCollection 2017 Jan-Mar. J Intercult Ethnopharmacol. 2017. PMID: 28163969 Free PMC article.
-
Bis(9)-(-)-Meptazinol, a novel dual-binding AChE inhibitor, rescues cognitive deficits and pathological changes in APP/PS1 transgenic mice.Transl Neurodegener. 2018 Sep 11;7:21. doi: 10.1186/s40035-018-0126-8. eCollection 2018. Transl Neurodegener. 2018. PMID: 30237878 Free PMC article.
-
Effects of BIS-MEP on Reversing Amyloid Plaque Deposition and Spatial Learning and Memory Impairments in a Mouse Model of β-Amyloid Peptide- and Ibotenic Acid-Induced Alzheimer's Disease.Front Aging Neurosci. 2019 Jan 22;11:3. doi: 10.3389/fnagi.2019.00003. eCollection 2019. Front Aging Neurosci. 2019. PMID: 30723404 Free PMC article.
-
Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer's disease.Bioorg Chem. 2021 Mar;108:104681. doi: 10.1016/j.bioorg.2021.104681. Epub 2021 Jan 29. Bioorg Chem. 2021. PMID: 33571811 Free PMC article. Review.
-
A Trishistidine Pseudopeptide with Ability to Remove Both CuΙ and CuΙΙ from the Amyloid-β Peptide and to Stop the Associated ROS Formation.Chemistry. 2017 Dec 1;23(67):17078-17088. doi: 10.1002/chem.201703429. Epub 2017 Nov 9. Chemistry. 2017. PMID: 28846165 Free PMC article.
References
-
- Qiu Y., Wu X. J., Chen H. Z. Simultaneous changes in secretory amyloid precursor protein and beta-amyloid peptide release from rat hippocampus by activation of muscarinic receptors. Neurosci. Lett. 2014;352(1):41–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
