Presence and stability of rotors in atrial fibrillation: evidence and therapeutic implications
- PMID: 26786157
- PMCID: PMC4777913
- DOI: 10.1093/cvr/cvw011
Presence and stability of rotors in atrial fibrillation: evidence and therapeutic implications
Abstract
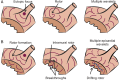
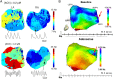
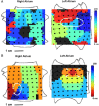
Rotor-guided ablation has opened new perspectives into the therapy of atrial fibrillation (AF). Analysis of the spatio-temporal cardiac excitation patterns in the frequency and phase domains has demonstrated the importance of rotors in research models of AF, however, the dynamics and role of rotors in human AF are still controversial. In this review, the current knowledge gained through research models and patient data that support the notion that rotors are key players in AF maintenance is summarized. We report and discuss discrepancies regarding rotor prevalence and stability in various studies, which can be attributed in part to methodological differences among mapping systems. Future research for validation and improvement of current clinical electrophysiology mapping technologies will be crucial for developing mechanistic-based selection and application of the best therapeutic strategy for individual AF patient, being it, pharmaceutical, ablative, or other approach.
Keywords: Atrial fibrillation; Body surface mapping; Dominant frequency; Fourier transform; Phase mapping; Rotors.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2016. For permissions please email: journals.permissions@oup.com.
Figures






References
-
- Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA 3rd, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, Stevenson WG, Tracy CM, Writing Group M, Fuster V, Ryden LE, Cannom DS, Le Heuzey JY, Crijns HJ, Lowe JE, Curtis AB, Olsson S, Ellenbogen KA, Prystowsky EN, Halperin JL, Tamargo JL, Kay GN, Wann L, Writing Committee M, Jacobs AK, Anderson JL, Albert N, Hochman JS, Buller CE, Kushner FG, Creager MA, Ohman EM, Ettinger SM, Stevenson WG, Guyton RA, Tarkington LG, Halperin JL, Yancy CW, Members AATF. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2011;123:104–123. - PubMed
-
- Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet 2010;375:1212–1223. - PubMed
-
- Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA, ThermoCool AFTI. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–340. - PubMed
-
- Parkash R, Tang AS, Sapp JL, Wells G. Approach to the catheter ablation technique of paroxysmal and persistent atrial fibrillation: a meta-analysis of the randomized controlled trials. J Cardiovasc Electrophysiol 2011;22:729–738. - PubMed
-
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

