A two-stage approach to genetic risk assessment in primary care
- PMID: 26786860
- PMCID: PMC4742331
- DOI: 10.1007/s10549-016-3686-2
A two-stage approach to genetic risk assessment in primary care
Abstract
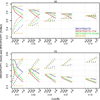
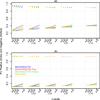
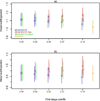
Genetic risk prediction models such as BRCAPRO are used routinely in genetic counseling for identification of potential BRCA1 and BRCA2 mutation carriers. They require extensive information on the counselee and her family history, and thus are not practical for primary care. To address this gap, we develop and test a two-stage approach to genetic risk assessment by balancing the tradeoff between the amount of information used and accuracy achieved. The first stage is intended for primary care wherein limited information is collected and analyzed using a simplified version of BRCAPRO. If the assessed risk is sufficiently high, more extensive information is collected and the full BRCAPRO is used (stage two: intended for genetic counseling). We consider three first-stage tools: BRCAPROLYTE, BRCAPROLYTE-Plus, and BRCAPROLYTE-Simple. We evaluate the two-stage approach on independent clinical data on probands with family history of breast and ovarian cancers, and BRCA genetic test results. These include population-based data on 1344 probands from Newton-Wellesley Hospital and mostly high-risk family data on 2713 probands from Cancer Genetics Network and MD Anderson Cancer Center. We use discrimination and calibration measures, appropriately modified to evaluate the overall performance of a two-stage approach. We find that the proposed two-stage approach has very limited loss of discrimination and comparable calibration as BRCAPRO. It identifies a similar number of carriers without requiring a full family history evaluation on all probands. We conclude that the two-stage approach allows for practical large-scale genetic risk assessment in primary care.
Keywords: BRCA1; BRCA2; BRCAPRO; BayesMendel; CancerGene.
Conflict of interest statement
Conflict of Interest
KH is a founder of and has a financial interest in Hughes Risk Apps (HRA), LLC. Dr. Hughes’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. KH has received honoraria from Myriad Genetics Speaker’s Bureau and holds stock options in 5 AM solutions. GP is on the SAB of HRA and holds stock options in the company.
Figures

References
-
- King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. - PubMed
-
- Drohan B, Roche CA, Cusack JC, Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. 2012;19:1732–1737. 2012. - PubMed
-
- Drohan B, Ozanne EM, Hughes KS. Electronic health records and the management of women at high risk of hereditary breast and ovarian cancer. Breast J. 2009;15(Suppl 1):46–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

