Does Valproic Acid or Levetiracetam Improve Survival in Glioblastoma? A Pooled Analysis of Prospective Clinical Trials in Newly Diagnosed Glioblastoma
- PMID: 26786929
- PMCID: PMC5070573
- DOI: 10.1200/JCO.2015.63.6563
Does Valproic Acid or Levetiracetam Improve Survival in Glioblastoma? A Pooled Analysis of Prospective Clinical Trials in Newly Diagnosed Glioblastoma
Abstract
Purpose: Symptomatic epilepsy is a common complication of glioblastoma and requires pharmacotherapy. Several uncontrolled retrospective case series and a post hoc analysis of the registration trial for temozolomide indicated an association between valproic acid (VPA) use and improved survival outcomes in patients with newly diagnosed glioblastoma.
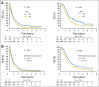
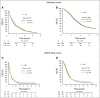
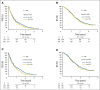
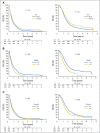
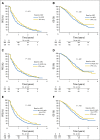
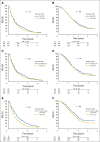
Patients and methods: To confirm the hypothesis suggested above, a combined analysis of survival association of antiepileptic drug use at the start of chemoradiotherapy with temozolomide was performed in the pooled patient cohort (n = 1,869) of four contemporary randomized clinical trials in newly diagnosed glioblastoma: AVAGlio (Avastin in Glioblastoma; NCT00943826), CENTRIC (Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Methylated Gene Promoter Status; NCT00689221), CORE (Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Unmethylated Gene Promoter Status; NCT00813943), and Radiation Therapy Oncology Group 0825 (NCT00884741). Progression-free survival (PFS) and overall survival (OS) were compared between: (1) any VPA use and no VPA use at baseline or (2) VPA use both at start of and still after chemoradiotherapy. Results of Cox regression models stratified by trial and adjusted for baseline prognostic factors were analyzed. The same analyses were performed with levetiracetam (LEV).
Results: VPA use at start of chemoradiotherapy was not associated with improved PFS or OS compared with all other patients pooled (PFS: hazard ratio [HR], 0.91; 95% CI, 0.77 to 1.07; P = .241; OS: HR, 0.96; 95% CI, 0.80 to 1.15; P = .633). Furthermore, PFS and OS of patients taking VPA both at start of and still after chemoradiotherapy were not different from those without antiepileptic drug use at both time points (PFS: HR, 0.92; 95% CI, 0.74 to 1.15; P = .467; OS: HR, 1.10; 95% CI, 0.86 to 1.40; P = .440). Similarly, no association with improved outcomes was observed for LEV use.
Conclusion: The results of this analysis do not justify the use of VPA or LEV for reasons other than seizure control in patients with newly diagnosed glioblastoma outside clinical trials.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures


Comment in
-
Valproate in Adjuvant Glioblastoma Treatment.J Clin Oncol. 2016 Sep 1;34(25):3105-7. doi: 10.1200/JCO.2016.67.2162. Epub 2016 Jun 13. J Clin Oncol. 2016. PMID: 27298401 No abstract available.
-
Reply to F. Felix et al and M.F. Fay et al.J Clin Oncol. 2016 Sep 1;34(25):3107-8. doi: 10.1200/JCO.2016.68.0926. Epub 2016 Jun 13. J Clin Oncol. 2016. PMID: 27298403 No abstract available.
-
Valproic Acid May Be Tested in Patients With H3F3A-Mutated High-Grade Gliomas.J Clin Oncol. 2016 Sep 1;34(25):3104-5. doi: 10.1200/JCO.2016.67.1073. Epub 2016 Jun 13. J Clin Oncol. 2016. PMID: 27298407 No abstract available.
References
-
- de Groot M, Reijneveld JC, Aronica E, et al. Epilepsy in patients with a brain tumour: Focal epilepsy requires focused treatment. Brain. 2012;135:1002–1016. - PubMed
-
- van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. - PubMed
-
- Weller M, Stupp R, Wick W. Epilepsy meets cancer: When, why, and what to do about it? Lancet Oncol. 2012;13:e375–e382. - PubMed
-
- Johannessen Landmark C, Johannessen SI, Tomson T. Host factors affecting antiepileptic drug delivery-pharmacokinetic variability. Adv Drug Deliv Rev. 2012;64:896–910. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

