Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V
- PMID: 26786933
- PMCID: PMC4933127
- DOI: 10.1200/JCO.2015.62.3504
Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V
Abstract
Purpose: Predicting the pattern of recurrence can aid in the development of targeted surveillance and treatment strategies. We identified patient populations that remain at risk for an event at a median follow-up of 24 years from the diagnosis of operable breast cancer.
Patients and methods: International Breast Cancer Study Group clinical trials I to V randomly assigned 4,105 patients between 1978 and 1985. Annualized hazards were estimated for breast cancer-free interval (primary end point), disease-free survival, and overall survival.
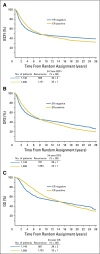
Results: For the entire group, the annualized hazard of recurrence was highest during the first 5 years (10.4%), with a peak between years 1 and 2 (15.2%). During the first 5 years, patients with estrogen receptor (ER)--positive disease had a lower annualized hazard compared with those with ER-negative disease (9.9% v 11.5%; P = .01). However, beyond 5 years, patients with ER-positive disease had higher hazards (5 to 10 years: 5.4% v 3.3%; 10 to 15 years: 2.9% v 1.3%; 15 to 20 years: 2.8% v 1.2%; and 20 to 25 years: 1.3% v 1.4%; P < .001). Among patients with ER-positive disease, annualized hazards of recurrence remained elevated and fairly stable beyond 10 years, even for those with no axillary involvement (2.0%, 2.1%, and 1.1% for years 10 to 15, 15 to 20, and 20 to 25, respectively) and for those with one to three positive nodes (3.0%, 3.5%, and 1.5%, respectively).
Conclusion: Patients with ER-positive breast cancer maintain a significant recurrence rate during extended follow up. Strategies for follow up and treatments to prevent recurrences may be most efficiently applied and studied in patients with ER-positive disease followed for a long period of time.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures


Comment in
-
Sisyphean Efforts: Establishing the Correct Risk-Benefit Balance for Adjuvant Therapies.J Clin Oncol. 2016 Mar 20;34(9):895-7. doi: 10.1200/JCO.2015.65.2255. Epub 2016 Jan 19. J Clin Oncol. 2016. PMID: 26786914 No abstract available.
References
-
- Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. - PubMed
-
- Goldhirsch A, Gelber RD, Price KN, et al. Effect of systemic adjuvant treatment on first sites of breast cancer relapse. Lancet. 1994;343:377–381. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

