Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis
- PMID: 26787172
- PMCID: PMC4772843
- DOI: 10.1093/cid/civ948
Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis
Abstract
Background: Treatment for hepatitis C virus (HCV) can lead to sustained virological response (SVR) in over 90% of people. Subsequent recurrence of HCV, either from late relapse or reinfection, reverses the beneficial effects of SVR.
Methods: A search identified studies analysing HCV recurrence post-SVR. The recurrence rate for each study was calculated using events/person years of follow-up (PYFU). Results were pooled using a random-effects model and used to calculate 5-year recurrence risk. Three patient groups were analysed: (1) Mono-HCV infected "low-risk" patients; (2) Mono-HCV infected "high-risk" patients (injecting drug users or prisoners); (3) human immunodeficiency virus (HIV)/HCV coinfected patients. Recurrence was defined as confirmed HCV RNA detectability post-SVR.
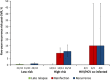
Results: In the 43 studies of HCV mono-infected "low-risk" patients (n = 7969) the pooled recurrence rate was 1.85/1000 PYFU (95% confidence interval [CI], .71-3.35; I(2) = 73%) leading to a summary 5-year recurrence risk of 0.95% (95% CI, .35%-1.69%). For the 14 studies of HCV monoinfected "high-risk" patients (n = 771) the pooled recurrence rate was 22.32/1000 PYFU (95% CI, 13.07-33.46; I(2) = 27%) leading to a summary 5-year risk of 10.67% (95% CI, 6.38%-15.66%). For the 4 studies of HIV/HCV coinfected patients the pooled recurrence rate was 32.02/1000 PYFU (95% CI, .00-123.49; I(2) = 96%) leading to a summary 5-year risk of 15.02% (95% CI, .00%-48.26%). The higher pooled estimates of recurrence in the high-risk and coinfected cohorts were driven by an increase in reinfection rather than late relapse.
Conclusions: SVR appears durable in the majority of patients at 5 years post-treatment. The large difference in 5 year event rate by risk group is driven mainly by an increased reinfection risk.
Keywords: hepatitis C; recurrence; reinfection; relapse; sustained virologic response.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Hepatitis C Virus Relapse 78 Weeks After Completion of Successful Direct-Acting Therapy.Clin Infect Dis. 2017 Sep 15;65(6):1051-1053. doi: 10.1093/cid/cix457. Clin Infect Dis. 2017. PMID: 28510632 No abstract available.
Similar articles
-
Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation.Health Technol Assess. 2011 Apr;15(17):i-xii, 1-210. doi: 10.3310/hta15170. Health Technol Assess. 2011. PMID: 21473834 Free PMC article.
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated.
-
The risk of hepatitis C virus recurrence in hepatitis C virus-infected patients treated with direct-acting antivirals after achieving a sustained virological response: A comprehensive analysis.Liver Int. 2021 Oct;41(10):2341-2357. doi: 10.1111/liv.14976. Epub 2021 Jun 16. Liver Int. 2021. PMID: 34051040 Free PMC article.
-
Reinfection and Resistance Associated Substitutions Following a Minimal Monitoring Approach for Hepatitis C Virus Treatment in MINMON Trial.Clin Infect Dis. 2025 Jul 18;80(6):1293-1301. doi: 10.1093/cid/ciae627. Clin Infect Dis. 2025. PMID: 39702964 Clinical Trial.
-
Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow-responder adult patients.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD008516. doi: 10.1002/14651858.CD008516.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972122 Free PMC article.
Cited by
-
Hepatitis C treatment uptake and response among human immunodeficiency virus/hepatitis C virus-coinfected patients in a large integrated healthcare system.Int J STD AIDS. 2019 Jun;30(7):689-695. doi: 10.1177/0956462419836520. Epub 2019 May 2. Int J STD AIDS. 2019. PMID: 31046611 Free PMC article.
-
Planning the hepatitis C virus elimination in Cyprus: A modeling study.World J Gastroenterol. 2021 Aug 21;27(31):5219-5231. doi: 10.3748/wjg.v27.i31.5219. World J Gastroenterol. 2021. PMID: 34497446 Free PMC article.
-
Direct-acting antiviral agents for HCV infection affecting people who inject drugs.Nat Rev Gastroenterol Hepatol. 2017 Nov;14(11):641-651. doi: 10.1038/nrgastro.2017.106. Epub 2017 Aug 23. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28831184 Review.
-
Path from the discovery to the elimination of hepatitis C virus: Honoring the winners of the Nobel Prize in Physiology or Medicine 2020.Kaohsiung J Med Sci. 2021 Jan;37(1):7-11. doi: 10.1002/kjm2.12345. Epub 2020 Dec 18. Kaohsiung J Med Sci. 2021. PMID: 33337581 Free PMC article. Review.
-
Relapse or reinfection after failing hepatitis C direct acting antiviral treatment: Unravelled by phylogenetic analysis.PLoS One. 2018 Jul 25;13(7):e0201268. doi: 10.1371/journal.pone.0201268. eCollection 2018. PLoS One. 2018. PMID: 30044871 Free PMC article.
References
-
- Gerlach J, Diepolder H, Zachoval R et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology 2003; 125:80–8. - PubMed
-
- Thein H, Yi Q, Dore G, Krahn M. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418–31. - PubMed
-
- Mohd Hanafiah K, Groeger J, Flaxman A, Wiersma S. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–42. - PubMed
-
- Pearlman B, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011; 52:889–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

