Protocol for a Prospective (P) study to develop a model to stratify the risk (RI) of medication (M) related harm in hospitalized elderly (E) patients in the UK (The PRIME study)
- PMID: 26787530
- PMCID: PMC4719738
- DOI: 10.1186/s12877-016-0191-8
Protocol for a Prospective (P) study to develop a model to stratify the risk (RI) of medication (M) related harm in hospitalized elderly (E) patients in the UK (The PRIME study)
Abstract
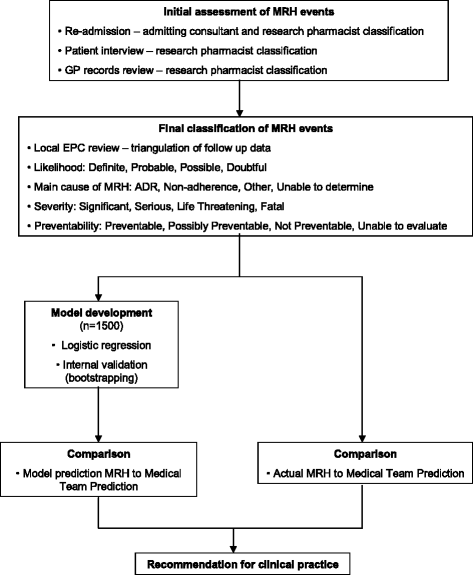
Background: Medication related harm (MRH) is a common cause of morbidity and hospital admission in the elderly, and has significant cost implications for both primary and secondary healthcare resources. The development of risk prediction models has become an increasingly common phenomenon in medicine and can be useful to guide objective clinical decision making, resource allocation and intervention. There are no risk prediction models that are widely used in clinical practice to identify elderly patients at high risk of MRH following hospital discharge. The aim of this study is to develop a risk prediction model (RPM) to identify elderly patients at high risk of MRH upon discharge from hospital, and to compare this with routine clinical judgment.
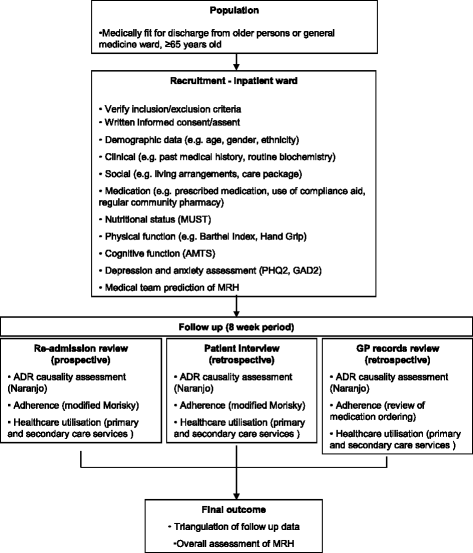
Methods/design: This is a multi-centre, prospective observational study following a cohort of patients for 8 weeks after hospital discharge. Data collection including patient characteristics, medication use, social factors and frailty will take place prior to patient discharge and then the patient will be followed up in the community over the next 8 weeks to determine if they have experienced MRH. Research pharmacists will determine whether patients have experienced MRH by prospectively reviewing records for unplanned emergency department attendance, hospital readmission and GP consultation related to MRH. Research pharmacists will also telephone patients directly to determine self-reported MRH, which patients may not have sought further medical attention for. The data collected will inform the development of a RPM which will be externally validated in a follow-up study.
Discussion: There are no RPMs that are used in clinical practice to help stratify elderly patients at high risk of MRH in the community following hospital discharge, despite this being a significant public health problem. This study plans to develop a clinically useful RPM that is better than routine clinical judgment. As this is a multi-centre study involving clinical settings that serve elderly people of heterogeneous sociodemographic background, it is anticipated that this RPM will be generalizable.
Figures
References
-
- Tangiisuran B, Gozzoli MP, Davies JG, Rajkumar C. Adverse drug reactions in older people. Rev Clin Gerontol. 2010;20:246–59. doi: 10.1017/S0959259810000171. - DOI
-
- Tangiisuran B, Davies JG, Wright JE, Rajkumar C. Adverse drug reactions in a population of hospitalized very elderly patients. Drugs Aging. 2012;29(8):669–79. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

