Synthetic α-Hydroxytropolones Inhibit Replication of Wild-Type and Acyclovir-Resistant Herpes Simplex Viruses
- PMID: 26787704
- PMCID: PMC4808205
- DOI: 10.1128/AAC.02675-15
Synthetic α-Hydroxytropolones Inhibit Replication of Wild-Type and Acyclovir-Resistant Herpes Simplex Viruses
Abstract
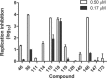
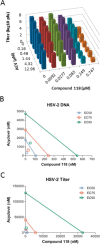
Herpes simplex virus 1 (HSV-1) and HSV-2 remain major human pathogens despite the development of anti-HSV therapeutics as some of the first antiviral drugs. Current therapies are incompletely effective and frequently drive the evolution of drug-resistant mutants. We recently determined that certain natural troponoid compounds such as β-thujaplicinol readily suppress HSV-1 and HSV-2 replication. Here, we screened 26 synthetic α-hydroxytropolones with the goals of determining a preliminary structure-activity relationship for the α-hydroxytropolone pharmacophore and providing a starting point for future optimization studies. Twenty-five compounds inhibited HSV-1 and HSV-2 replication at 50 μM, and 10 compounds inhibited HSV-1 and HSV-2 at 5 μM, with similar inhibition patterns and potencies against both viruses being observed. The two most powerful inhibitors shared a common biphenyl side chain, were capable of inhibiting HSV-1 and HSV-2 with a 50% effective concentration (EC50) of 81 to 210 nM, and also strongly inhibited acyclovir-resistant mutants. Moderate to low cytotoxicity was observed for all compounds (50% cytotoxic concentration [CC50] of 50 to >100 μM). Therapeutic indexes ranged from >170 to >1,200. These data indicate that troponoids and specifically α-hydroxytropolones are a promising lead scaffold for development as anti-HSV drugs provided that toxicity can be further minimized. Troponoid drugs are envisioned to be employed alone or in combination with existing nucleos(t)ide analogs to suppress HSV replication far enough to prevent viral shedding and to limit the development of or treat nucleos(t)ide analog-resistant mutants.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Roizman B, Knipe DM, Whitley RJ. 2013. Herpes simplex viruses, p 1823–1897. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi:10.1056/NEJMoa1103151. - DOI - PMC - PubMed
-
- Bernstein DI, Bellamy AR, Hook EW III, Levin MJ, Wald A, Ewell MG, Wolff PA, Deal CD, Heineman TC, Dubin G, Belshe RB. 2013. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 56:344–351. doi:10.1093/cid/cis891. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

