Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab
- PMID: 26787752
- PMCID: PMC5770142
- DOI: 10.1158/1078-0432.CCR-15-2412
Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab
Abstract
Purpose: To identify baseline peripheral blood biomarkers associated with clinical outcome following ipilimumab treatment in advanced melanoma patients.
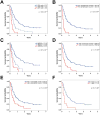
Experimental design: Frequencies of myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg), serum lactate dehydrogenase (LDH), routine blood counts, and clinical characteristics were assessed in 209 patients. Endpoints were overall survival (OS) and best overall response. Statistical calculations were done by Kaplan-Meier and Cox regression analysis, including calibration and discrimination by C-statistics.
Results: Low baseline LDH, absolute monocyte counts (AMC), Lin(-)CD14(+)HLA-DR(-/low)-MDSC frequencies, and high absolute eosinophil counts (AEC), relative lymphocyte counts (RLC), and CD4(+)CD25(+)FoxP3(+)-Treg frequencies were significantly associated with better survival, and were considered in a combination model. Patients (43.5%) presenting with the best biomarker signature had a 30% response rate and median survival of 16 months. In contrast, patients with the worst biomarkers (27.5%) had only a 3% response rate and median survival of 4 months. The occurrence of adverse events correlated with neither baseline biomarker signatures nor the clinical benefit of ipilimumab. In another model, limited to the routine parameters LDH, AMC, AEC, and RLC, the number of favorable factors (4 vs. 3 vs. 2-0) was also associated with OS (P < 0.001 for all pairwise comparisons) in the main study and additionally in an independent validation cohort.
Conclusions: A baseline signature of low LDH, AMC, and MDSCs as well as high AEC, Tregs, and RLC is associated with favorable outcome following ipilimumab. Prospective investigation of the predictive impact of these markers following ipilimumab and other treatments, e.g., PD-1 antibodies, is warranted. Clin Cancer Res; 22(12); 2908-18. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed by the other authors
Figures

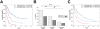
References
-
- Robert C, Thomas L, Bondarenko I, O’Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. - PubMed
-
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. - PubMed
-
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

