Insights into the Function of a Second, Nonclassical Ahp Peroxidase, AhpA, in Oxidative Stress Resistance in Bacillus subtilis
- PMID: 26787766
- PMCID: PMC4800881
- DOI: 10.1128/JB.00679-15
Insights into the Function of a Second, Nonclassical Ahp Peroxidase, AhpA, in Oxidative Stress Resistance in Bacillus subtilis
Abstract
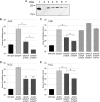
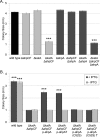
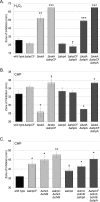
Organisms growing aerobically generate reactive oxygen-containing molecules, such as hydrogen peroxide (H2O2). These reactive oxygen molecules damage enzymes and DNA and may even cause cell death. In response, Bacillus subtilis produces at least nine potential peroxide-scavenging enzymes, two of which appear to be the primary enzymes responsible for detoxifying peroxides during vegetative growth: a catalase (encoded by katA) and an alkylhydroperoxide reductase (Ahp, encoded by ahpC). AhpC uses two redox-active cysteine residues to reduce peroxides to nontoxic molecules. A specialized thioredoxin-like protein, AhpF, is then required to restore oxidized AhpC back to its reduced state. Curiously, B. subtilis has two genes encoding Ahp: ahpC and ahpA. Although AhpC is well characterized, very little is known about AhpA. In fact, numerous bacterial species have multiple ahp genes; however, these additional Ahp proteins are generally uncharacterized. We seek to understand the role of AhpA in the bacterium's defense against toxic peroxide molecules in relation to the roles previously assigned to AhpC and catalase. Our results demonstrate that AhpA has catalytic activity similar to that of the primary enzyme, AhpC. Furthermore, our results suggest that a unique thioredoxin redox protein, AhpT, may reduce AhpA upon its oxidation by peroxides. However, unlike AhpC, which is expressed well during vegetative growth, our results suggest that AhpA is expressed primarily during postexponential growth.
Importance: B. subtilis appears to produce nine enzymes designed to protect cells against peroxides; two belong to the Ahp class of peroxidases. These studies provide an initial characterization of one of these Ahp homologs and demonstrate that the two Ahp enzymes are not simply replicates of each other, suggesting that they instead are expressed at different times during growth of the cells. These results highlight the need to further study the Ahp homologs to better understand how they differ from one another and to identify their function, if any, in protection against oxidative stress. Through these studies, we may better understand why bacteria have multiple enzymes designed to scavenge peroxides and thus have a more accurate understanding of oxidative stress resistance.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

