Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate-to-severe pain after total hip arthroplasty
- PMID: 26787797
- PMCID: PMC4718147
- DOI: 10.1093/bja/aev457
Randomized clinical trial of dexketoprofen/tramadol 25 mg/75 mg in moderate-to-severe pain after total hip arthroplasty
Abstract
Background: The aim was to evaluate the analgesic efficacy and safety of the dexketoprofen/tramadol 25 mg/75 mg fixed-dose combination vs dexketoprofen (25 mg) and tramadol (100 mg) in moderate-to-severe acute pain after total hip arthroplasty.
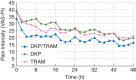
Methods: This was a randomized, double-blind, parallel-group study in patients experiencing pain of at least moderate intensity on the day after surgery, compared with placebo at first administration to validate the pain model. The study drug was administered orally every 8 h throughout a 5 day period. Rescue medication, metamizole 500 mg, was available during the treatment period. The evaluation of efficacy was based on patient assessments of pain intensity and pain relief. The primary end point was the mean sum of the pain intensity difference values throughout the first 8 h (SPID8).
Results: Overall, 641 patients, mean age 62 (range 29-80) yr, were analysed; mean (sd) values of SPID8 were 247 (157) for dexketoprofen/tramadol, 209 (155) for dexketoprofen, 205 (146) for tramadol, and 151 (159) for placebo. The primary analysis confirmed the superiority of the combination over dexketoprofen 25 mg (P=0.019; 95% confidence interval 6.4-73) and tramadol 100 mg (P=0.012; 95% confidence interval 9.5-76). The single components were superior to placebo (P<0.05), confirming model sensitivity. Most secondary analyses supported the superiority of the combination. The incidence of adverse drug reactions was low and similar among active treatment groups.
Conclusion: The efficacy results confirmed the superiority of dexketoprofen/tramadol over its single components, even at higher doses (tramadol), with a safety profile fully in line with that previously known for these agents in monotherapy.
Clinical trial registration: EudraCT 2012-004548-31 (https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2012-004548-31);ClinicalTrials.gov NCT01902134 (https://www.clinicaltrials.gov/ct2/show/NCT01902134?term=NCT01902134&rank=1).
Keywords: analgesics; arthroplasty, replacement, hip; dexketoprofen trometamol; pain, postoperative; tramadol.
© The Author 2016. Published by Oxford University Press on behalf of the British Journal of Anaesthesia.
Figures


References
-
- Curatolo M, Sveticic G. Drug combinations in pain treatment: a review of the published evidence and a method for finding the optimal combination. Best Pract Res Clin Anaesthesiol 2002; 16: 507–19 - PubMed
-
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002; 183: 630–41 - PubMed
-
- Hartrick CT. Multimodal post-operative pain management. Am J Health Syst Pharm 2004; 61(Suppl 1): S4–10 - PubMed
-
- European Medicines Agency. Note for guidance on clinical investigation of medicinal products for treatment of nociceptive pain (CPMP/EWP/612/00). Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (accessed 15 April 2015)
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

