Multiscale model of light harvesting by photosystem II in plants
- PMID: 26787911
- PMCID: PMC4747709
- DOI: 10.1073/pnas.1524999113
Multiscale model of light harvesting by photosystem II in plants
Abstract
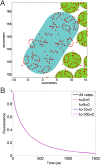
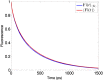
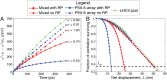
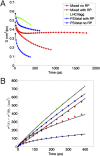
The first step of photosynthesis in plants is the absorption of sunlight by pigments in the antenna complexes of photosystem II (PSII), followed by transfer of the nascent excitation energy to the reaction centers, where long-term storage as chemical energy is initiated. Quantum mechanical mechanisms must be invoked to explain the transport of excitation within individual antenna. However, it is unclear how these mechanisms influence transfer across assemblies of antenna and thus the photochemical yield at reaction centers in the functional thylakoid membrane. Here, we model light harvesting at the several-hundred-nanometer scale of the PSII membrane, while preserving the dominant quantum effects previously observed in individual complexes. We show that excitation moves diffusively through the antenna with a diffusion length of 50 nm until it reaches a reaction center, where charge separation serves as an energetic trap. The diffusion length is a single parameter that incorporates the enhancing effect of excited state delocalization on individual rates of energy transfer as well as the complex kinetics that arise due to energy transfer and loss by decay to the ground state. The diffusion length determines PSII's high quantum efficiency in ideal conditions, as well as how it is altered by the membrane morphology and the closure of reaction centers. We anticipate that the model will be useful in resolving the nonphotochemical quenching mechanisms that PSII employs in conditions of high light stress.
Keywords: excitation energy transfer; fluorescence lifetime; photosynthesis; quantum coherence; structure−function relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Blankenship RE. Molecular Mechanisms of Photosynthesis. 2nd Ed Blackwell Sci; Malden, MA: 2014.
-
- Baker NR. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89–113. - PubMed
-
- Ruban AV, Johnson MP, Duffy CDP. The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta. 2012;1817(1):167–181. - PubMed
-
- Külheim C, Ågren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science. 2002;297(5578):91–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

