Electrical Stimulation Promotes Cardiac Differentiation of Human Induced Pluripotent Stem Cells
- PMID: 26788064
- PMCID: PMC4691644
- DOI: 10.1155/2016/1718041
Electrical Stimulation Promotes Cardiac Differentiation of Human Induced Pluripotent Stem Cells
Abstract
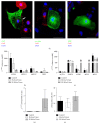
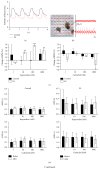
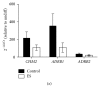
Background. Human induced pluripotent stem cells (iPSCs) are an attractive source of cardiomyocytes for cardiac repair and regeneration. In this study, we aim to determine whether acute electrical stimulation of human iPSCs can promote their differentiation to cardiomyocytes. Methods. Human iPSCs were differentiated to cardiac cells by forming embryoid bodies (EBs) for 5 days. EBs were then subjected to brief electrical stimulation and plated down for 14 days. Results. In iPS(Foreskin)-2 cell line, brief electrical stimulation at 65 mV/mm or 200 mV/mm for 5 min significantly increased the percentage of beating EBs present by day 14 after plating. Acute electrical stimulation also significantly increased the cardiac gene expression of ACTC1, TNNT2, MYH7, and MYL7. However, the cardiogenic effect of electrical stimulation was not reproducible in another iPS cell line, CERA007c6. Beating EBs from control and electrically stimulated groups expressed various cardiac-specific transcription factors and contractile muscle markers. Beating EBs were also shown to cycle calcium and were responsive to the chronotropic agents, isoproterenol and carbamylcholine, in a concentration-dependent manner. Conclusions. Our results demonstrate that brief electrical stimulation can promote cardiac differentiation of human iPS cells. The cardiogenic effect of brief electrical stimulation is dependent on the cell line used.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

